��Ŀ����
8��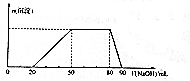 ij50 mL��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Al3+��SO42-�����ӣ��������Һ�м���5 mol•L-1NaOH��Һʱ���������ɳ��������ʵ���n����������NaOH��Һ�����V��NaOH���仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
ij50 mL��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Al3+��SO42-�����ӣ��������Һ�м���5 mol•L-1NaOH��Һʱ���������ɳ��������ʵ���n����������NaOH��Һ�����V��NaOH���仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ԭ��Һ��һ������Mg2+��Al3+��H+��SO42- | |
| B�� | ԭ��Һ��Al3+��Ũ��Ϊ1mol•L-1 | |
| C�� | ԭ��Һ��NH4+�����ʵ���Ϊ0.4mol | |
| D�� | �������NaOH����Һ�����Ϊ90mLʱ����Ӧ����Һ�е�����ֻ��Na+��SO42- |
���� ��ʼ����NaOHû�г����������������һ����H+�������г��������������ʧ����һ��û��Mg2+��һ����Al3+���м�γ������������䣬ӦΪNH4++OH-=NH3•H2O�ķ�Ӧ������NH4+�������ǵõ���������������ȫ���ܽ⣬�������ӷ�Ӧ���������������жϼ�����ػ�ѧ����ʽ���м��㣮
��� �⣺��ͼ���֪����ʼʱ����������˵������H+��H++OH-=H2O��������������NaOH��Һ�����Ϊ20mL��ͼ������һ��ƽ̨��˵������OH-ʱ�������ɣ���NH4+��NH4++OH-=NH3•H2O��笠�������������Ϊ30mL�������Һ��������˵����Һ�в���Mg2+������Al3+������Al3++3OH-=Al��OH��3��������������NaOH��Һ�����Ϊ30mL��������40mL����������ʱ������ʧ�����Կɵõ�H+��Al3+��NH4+�����ʵ���֮��Ϊ2��1��3�����ݵ�����ԭ����һ������SO42-��
A��ԭ��Һ��һ������NH4+��Al3+��H+��SO42-����Һ�в���Mg2+����A����
B������Al3++3OH-=Al��OH��3����ԭ��Һ��Al3+�����ʵ�����0.03L��5 mol•L-1��$\frac{1}{3}$=0.05mol��Ũ��Ϊ$\frac{0.05mol}{0.05L}$=1mol•L-1����B��ȷ��
C�����ݷ�Ӧ��NH4++OH-=NH3•H2O��ԭ��Һ��NH4+�����ʵ���Ϊ0.03L��5 mol•L-1=0.15mol����C����
D���������NaOH����Һ�����Ϊ90mLʱ����Ӧ����Һ�е�������Na+��SO42-��AlO2-����D����
��ѡB��
���� ���⿼�����ӵ��жϺͼ��㣬��Ŀ�Ѷ��еȣ�Ҫ��ѧ��������ʵ����ʲ����Ӧ�ã�ע��ͼ������������ķ�Ӧ�ǹؼ���

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�| A�� | ������ˮ�����γ�Al��OH��3���壬��������ˮ�� | |
| B�� | ���ʯ����Ȼ����Ӳ���������ʣ�������������������Ӧ | |
| C�� | Ư�ۡ����ᡢ��ƺ�Һ�ȶ����ڻ���� | |
| D�� | ��SO2ͨ��Ʒ����Һ����Һ��ɫ����Ȼָ�ԭɫ����SO2ͨ����ˮ����ˮ��ɫ�����Ҳ�ָܻ�ԭɫ |
| A�� | [Cl-]��[NH4+]��[H+]��[OH-] | B�� | [NH4+]��[Cl-]��[OH-]��[H+] | C�� | [Cl-]��[NH4+]��[OH-]=[H+] | D�� | [Cl-]=[NH4+]��[H+]��[OH-] |
����Na2SiO3��Һ��ͨ��CO2������ɫ����
��ʯī�ǵ��壬������ǰ뵼��
���Ʋ��������з�����ӦNa2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2��
��CH4��SiH4�ȶ���
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �٢� |
| A�� | 0.1NA��NO��һ�ܱ�������������������ȫ��Ӧ���õ�0.1molNO2 | |
| B�� | һ�������µ��ܱ������У�6.4gSO2�ɱ����������õ�SO3�ķ�����Ϊ0.1NA | |
| C�� | ������18g����������Ũ������ת�Ƶ�����2NA | |
| D�� | 1L0.1mol/L FeCl3��Һ��ȫˮ��õ���Fe��OH��3��������С��0.1NA |
| A�� | HCl��NaOH��Ӧ���к��ȡ�H=-57.3 kJ•mol-1����H2SO4��Ca��OH��2��Ӧ���к��ȡ�H=2����-57.3��kJ•mol-1 | |
| B�� | CO��g����ȼ������283.0 kJ•mol-1����2CO2��g���T2CO��g��+O2��g���ġ�H=2����+283.0��kJ•mol-1 | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| D�� | 1 mol����ȼ��������̬ˮ�Ͷ�����̼���ų��������Ǽ����ȼ���� |
| A�� | �٢ڢ�����������ֱ���FeCl2��Fe3+��Cl2 | |
| B�� | �������Ϸ�Ӧ�Ļ�ѧ����ʽ���Եõ�������ǿ����ϵΪCl2��Co2O3��Fe3+ | |
| C�� | �ڷ�Ӧ��������1 mol Cl2ʱ����2 mol HCl������ | |
| D�� | ���ݷ�Ӧ�٢�һ�����������õ�Cl2+FeBr2�TFeCl2+Br2 |
��1������ĵ��뷽��ʽ��H2C2O4?HC2O4-+H+ HC2O4-?C2O42-+H+��
��2��Ũ��Ϊ0.1mol/L��Na2C2O4��Һ�У�c��C2O42-��+c��HC2O4-��+c��H2C2O4��=0.1mol/L
��3��40��ʱ���һ�������0.1mol/L H2C2O4��Һ��һ�����0.01mol/L����KMnO4��Һ����д���пո�
| �¶� | v��H2C2O4�� | v��KMnO4�� | KMnO4��ɫʱ�� |
| 40�� | 10mL | 10mL | 40s |
| 40�� | 20mL | 20mL |
��5��������MgC2O4��Ksp=8.1��10-5����֪����Һ��ij���ӵ�Ũ�ȡ�10-5mol/Lʱ����Ϊ�������ѳ�����ȫ����Ϊ�˳���1L0.01mol/LMgCl2��Һ�е�Mg2+������100mL0.1mol/L�ģ�NH4��2C2O4��Һ��ͨ�������ж�Mg2+�Ƿ��ѳ�����ȫ��д��������̣���