��Ŀ����
18���ڻ�ѧʵ���г���Ҫ�õ���Һ��ȷ����һ�����ʵ���Ũ�ȵ���Һ��Ҳ��һ�ֺܻ�����ʵ�������ʵ������Ҫ480mL0.1mol/L��NaOH��Һ��������Һ��������ش��������⣺��1��ʵ���г���������ƽ����Ͳ���ձ���ҩ�����Ҫ�IJ��������У���������500mL����ƿ����ͷ�ι�
��2�����ݼ����֪������NaOH������Ϊ2.0g
��3�����������Һ��ȡ��5mL���������ʵ���Ũ��Ϊ0.1mol/L���ټ�ˮϡ����100mL����ϡ�ͺ���Һ�����ʵ���Ũ��Ϊ0.005mol/L�����к�NaOH������Ϊ0.02g����100mL��Һ���Ը�c��H+��=0.1mol/L��������Һ2.5mL��ȫ�к�����Na2SO4��
��4�����в����ᵼ��������ҺŨ��ƫ�͵���ABEGI������ĸ��
A�����ձ����ܽ�ʱ��������Һ�彦�� B������ʱ��������ƿ�̶���
C������ƿʹ��ǰδ���� D������ʱ��������ƿ�̶���
E������NaOH�Ѿ����� F��������ƿ�м�ˮδ���̶���
G�����ù����ձ���������δϴ�� H��NaOH�ܽ��δ�������¼����ж���
I������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶������У�
���� ��1������������Һ�IJ�������ѡ������������
��2������m=CVM������Ҫ���ʵ�������
��3����Һ���о�һ�ԣ�������Һϡ�������������ʵ����ʵ����������ϡ�ͺ���Һ�����ʵ���Ũ�ȣ�����n=CVM���㺬���������Ƶ�����������2NaOH��H2SO4������������������
��4������c=$\frac{n}{V}$�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��� �⣺��1���ù�������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ���������������ƽ����Ͳ���ձ���ҩ�ס���������500mL����ƿ����ͷ�ιܣ����Ի�ȱ�ٵ���������������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����������500mL����ƿ����ͷ�ιܣ�
��2����Ҫ480mL0.1mol/L��NaOH��Һ��Ӧѡ��500mL����ƿ����Ҫ���ʵ�����Ϊ��0.1mol/L��0.5L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��3����Һ���о�һ�ԣ�0.1mol/L��NaOH��Һ����ȡ��5mL���������ʵ���Ũ��Ϊ 0.1mol/L���ټ�ˮϡ����100mL����ϡ�ͺ���Һ�����ʵ���Ũ��ΪC����C��100mL=0.1mol/L��5mL�����C=0.005mol/L�����������������Ƶ����ʵ���Ϊ0.1mol/L��0.005L��40g/mol=0.0005mol=0.02g������2NaOH��H2SO4�����к�0.0005mol�������ƣ���Ҫ��������ʵ���Ϊ$\frac{1}{2}$��0.0005mol=0.00025mol������Ҫc��H+��=0.1mol/L��������Һ���V=$\frac{0.00025mol}{0.1mol/L}$=0.0025L����2.5mL��
�ʴ�Ϊ��0.1mol/L��0.005mol/L��0.02g��2.5��
��4��A�����ձ����ܽ�ʱ��������Һ�彦�����������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Aѡ��
B������ʱ��������ƿ�̶��ߣ�������Һ���ƫ����ҺŨ��ƫС����Bѡ��
C������ƿʹ��ǰδ��������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬��C��ѡ��
D������ʱ��������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���D��ѡ��
E������NaOH�Ѿ����⣬���ȡ���������Ƶ�����ƫС����Һ��Ũ��ƫ�ͣ���Eѡ��
F��������ƿ�м�ˮδ���̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���F��ѡ��
G�����ù����ձ���������δϴ�ӣ��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Gѡ��
H��NaOH�ܽ��δ�������¼����ж��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���H��ѡ��
I������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶������� ���²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Iѡ��
�ʴ�Ϊ��ABEGI��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ŀ�Ѷ��еȣ�Ҫ��ѧ����������һ�����ʵ���Ũ����Һ����ȷ���������������������������뼼�ɣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�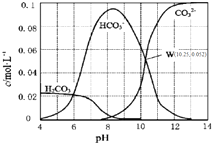 25��ʱ��0.1mol•L-1̼������Һ��ͨ��HCl���壬��̼���ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
25��ʱ��0.1mol•L-1̼������Һ��ͨ��HCl���壬��̼���ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��pH=7.0ʱ����Һ�к�̼����ֻ��CO32-��HCO3- | |
| B�� | ��Na2CO3��Һ��ͨ��HCl���壬��������CO2���� | |
| C�� | H2CO3��Ka2=1.0��10-10.25 | |
| D�� | ��100 mL 0.1 mol•L-1̼������Һ�еμ���������ҺpH=4.0������CO2����224 mL |
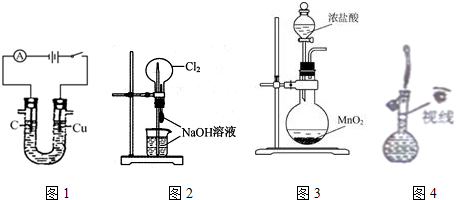
| A�� | ͼ1װ�ÿ���������������������� | |
| B�� | ͼ2װ�ÿ�����ɡ���Ȫ��ʵ�� | |
| C�� | ͼ3װ�ÿ�������ʵ���������� | |
| D�� | ͼ4װ�ÿ�����������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ |
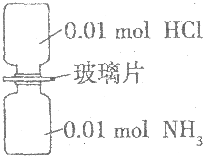 �����£���ȥ��ͼ��ʾװ���еIJ���Ƭ��ʹ���������ַ�Ӧ������˵����ȷ���ǣ���NA��ʾ�����ӵ���������ֵ����������
�����£���ȥ��ͼ��ʾװ���еIJ���Ƭ��ʹ���������ַ�Ӧ������˵����ȷ���ǣ���NA��ʾ�����ӵ���������ֵ����������| A�� | װ������Ԫ�ص�������Ϊ0.04g | |
| B�� | ����������������Ϊ0.448 L | |
| C�� | ���������0.01NA������ | |
| D�� | ��������ȫ����ˮ��������Һ����0.01NA��NH4Cl |
| A�� | ��ˮʹ��̪��Һ����ԭ��NH3•H2O�TNH4++OH- | |
| B�� | ����������Һ�����ԣ�NaHSO4?Na++H++SO42- | |
| C�� | ������ˮԭ����Al 3++3 H2O?Al��OH��3��+3 H+ | |
| D�� | �Ʊ�TiO2���ۣ�TiCl4+��x+2��H2O��������?TiO2•xH2O��+4 HCl |
| A�� | ��ij��Һ�еμӼ���˫��ˮ���ٵμ�2��KSCN��Һ����Һ���Ѫ��ɫ����ԭ��Һ��һ������Fe2+ | |
| B�� | ��ij��Һ�м��������ữ���Ȼ�����Һ��������ɫ��������ԭ��Һ��һ������SO42- | |
| C�� | ��ij��Һ�м���ϡ���������ɫ���壬����������ʹ����ʯ��ˮ����ǣ���ԭ��Һ��һ����CO32- | |
| D�� | ��ij��Һ�еμ�NaOH��Һ�����ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ��������ԭ��Һ��һ���� ��NH4+ |
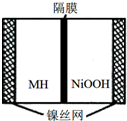 ��ͼ���ں����ø�ѹ������ػ����Ϸ�չ������һ�ֽ����⻯������أ�MH-Ni��أ����õ�طŵ�ʱ�ܷ�ӦΪ��NiOOH+MH�TNi��OH��2+M�������й�˵���в���ȷ���ǣ�������
��ͼ���ں����ø�ѹ������ػ����Ϸ�չ������һ�ֽ����⻯������أ�MH-Ni��أ����õ�طŵ�ʱ�ܷ�ӦΪ��NiOOH+MH�TNi��OH��2+M�������й�˵���в���ȷ���ǣ�������| A�� | �ŵ�ʱ������ӦΪ��NiOOH+H2O+e-�TNi��OH��2+OH- | |
| B�� | ��صĵ��Һ��ΪKOH��Һ | |
| C�� | ���ʱ������ӦΪ��MH+OH-+e-�TH2O+M | |
| D�� | MH��һ�ഢ����ϣ������ܶ�Խ��ص������ܶ�Խ�� |
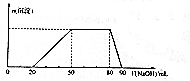 ij50 mL��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Al3+��SO42-�����ӣ��������Һ�м���5 mol•L-1NaOH��Һʱ���������ɳ��������ʵ���n����������NaOH��Һ�����V��NaOH���仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
ij50 mL��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Al3+��SO42-�����ӣ��������Һ�м���5 mol•L-1NaOH��Һʱ���������ɳ��������ʵ���n����������NaOH��Һ�����V��NaOH���仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ԭ��Һ��һ������Mg2+��Al3+��H+��SO42- | |
| B�� | ԭ��Һ��Al3+��Ũ��Ϊ1mol•L-1 | |
| C�� | ԭ��Һ��NH4+�����ʵ���Ϊ0.4mol | |
| D�� | �������NaOH����Һ�����Ϊ90mLʱ����Ӧ����Һ�е�����ֻ��Na+��SO42- |