��Ŀ����
4��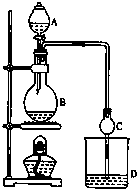 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
| �Լ� | �Ҵ� | ���� | �������� |
| �е�/�� | 78.5 | 118 | 77.1 |
��1��Ũ��������ã���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ���÷�Ӧԭ����д���ܱ�ʾ18O�ڷ�Ӧǰ��λ�ñ仯�Ļ�ѧ����ʽ��CH3COOH+CH3CH218OH
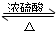 CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O����2�����θ����C�������Ƿ�ֹ������������
��3����D�з���������������г�����һ�������Ҵ�����ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ�������Ҵ����ټ��루�˿մ�����ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
���� ��1�������������Ҵ��ڼ��ȡ�Ũ�����������������·���������Ӧ���������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���
��2�����θ���ܵĹܿ�����Һ���¿��Է�ֹ������ͬʱ�����������ã�
���ڷֲ�õ�������D�е���ҺΪ���͵�̼������Һ��̼�������������������ˮ�⣬��Һ�ʼ��ԣ���Ӧ����Һ�ֲ㣬�ϲ���ɫ����Һ�壬����̼���������ᷴӦ�����²���Һ��ɫ�䣻
��3�������Ϣ��֪���Ҵ������������ķе�ӽ���������ķе��������ȼ�����ˮ�Ȼ��ƣ�������Ҵ���Ȼ������ˮ�����Ƴ�ȥ������ˮ��
��� �⣺��1���������Ҵ��ڼ��ȡ�Ũ�����������������·���������Ӧ���������������÷�ӦΪ���淴Ӧ������ʽΪ��CH3COOH+CH3CH218OH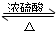 CH3CO18OC2H5+H2O��Ũ������ˮ����ƽ���������������������ƶ�����Ũ���������Ϊ��������ˮ����
CH3CO18OC2H5+H2O��Ũ������ˮ����ƽ���������������������ƶ�����Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����CH3COOH+CH3CH218OH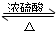 CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O��
��2�����θ���ܵĹܿ�����Һ���¿��Է�ֹ������ͬʱ�����������ã�
�ʴ�Ϊ����ֹ������������
��3�����Ȼ��Ƴ�ȥ�����Ҵ�������ˮ�����Ƴ�ȥ������ˮ����ˮ��������ˮ�γ������ƽᾧˮ�������ѡ��P2O5����ʯ�Һ�NaOH�ȹ����������Է��������������ԣ�P2O5��ˮ�����ᣩ�����������ˮ�⣻
�ʴ�Ϊ���Ҵ���C��
���� ���⿼���л�����Ʊ�ʵ�飬����ϰ���е���Ϣ���Ʊ�ԭ����ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��������뼰��Ϣ��Ӧ�ã���Ŀ�Ѷ��еȣ�

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д���1���ɻ��������������������������䷽�ж��֣�����Ҫ����Ϊ��ʯ�ң�Ca0�������Na2C03����ʳ�Σ�NaCl������һ��������������ˮ��������Ƴɺ�״�����ڵ��ϣ��ܷⱣ�棬���պ�ʳ�ã�
�ٽ��ɻ�����ĺ�״��������ˮ�ܽ⣬�ܽ�����з�Ӧ�Ļ�ѧ����ʽ�ֱ�ΪCaO+H2O=Ca��OH��2��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��ijͬѧ��������ʾ��ʵ�鷽����̽�����з�Ӧ�����������Һ�п��ܺ��е����ʣ������������ʵ�鷽����
| ʵ�鲽�� | ʵ������ | ���� |
| ȡ������Һ���μ�����K2CO3��Һ | �����ְ�ɫ���� | ��Һ�к�Ca��OH��2 |
| ���ް�ɫ���� | ��Һ����Ca��OH��2 | |
| ��ȡ������Һ�������������� | ���������� | ��Һ�к�Na2CO3 |

���Ȼ�����Һ���ܣ���ܡ����ܡ��������ᱵ��Һ���棬�����ǻ������µ����ʣ�
�ڼ���̼������Һ��Ŀ���dz�ȥ������Ba2+��Ϊʲô���ȹ��˶������̼������Һ��������һ���Թ��˳����ᱵ��̼�ᱵ�����������ظ�������

��1����д��ʵ������ȡ���������Ļ�ѧ����ʽCH3COOH+CH3CH2OH $?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O
��2�����ס��ҡ�������ʵ��װ���У�����ѡ�õ�װ���DZ� ����ס��ҡ�����
��3����������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ����ش�

�Լ�a�DZ���̼������Һ�����뷽�����Ƿ�Һ�����뷽����������
��4����Ϊ��֤��Ũ�����ڷ�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ijװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܼ����Լ� | �Թ������Լ� | �л���ĺ��/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol•L-1 Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol•L-1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
�ڷ���ʵ��AC����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʣ�
| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е㣨�棩 | 56.2 | 77.06 | 78 | 117.9 |
�����Һ�м����ռ���Һ��������Һ��pH=10
�ڽ����Һ�����������л�������
���ռ��¶���75��85��ʱ�������
����ȴ����������ƿ�м�Ũ���ᣨ��������Ȼ���ٽ��������������
��ش��������⣺
��1�������ռ�ʹ��Һ��pH=10��Ŀ���ǣ��û�ѧ����ʽ��ʾ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��2����75��85��ʱ��������Ҫ�ɷ����Ҵ������������ƣ���
��3���ڲ�����У��������Ũ�����Ŀ���ǽ�������ת��Ϊ���
��4�������������¶ȿ�����85��125��һ��ʱ�����������Ҫ�ɷ������ᣨ�����ƣ���
| A�� | �κ��������кͷ�Ӧ����1 mol H2O�Ĺ����У������仯����ͬ | |
| B�� | ͬ��ͬѹ�£�H2��g��+Cl2��g���T2HCl��g���ڹ��պ͵�ȼ�����µġ�H��ͬ | |
| C�� | ��ѧ��Ӧ���ջ�ų������Ķ�����μӷ�Ӧ�����ʵĶ����� | |
| D�� | C��ʯī��s���TC�����ʯ��s����H��0������ʯī�Ƚ��ʯ�ȶ� |
 H���ļ��ֱܷ�Ϊ436��496��462 kJ��mol��1���� a Ϊ
H���ļ��ֱܷ�Ϊ436��496��462 kJ��mol��1���� a Ϊ
 ����ͼ�������Թ����ȼ���2mL 95%���Ҵ�������ҡ���»�������3mLŨ���ᣬ�ټ���2mL���ᣬ���ҡ�ȣ������Թ��м���5mL����Na2CO3��Һ����ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮
����ͼ�������Թ����ȼ���2mL 95%���Ҵ�������ҡ���»�������3mLŨ���ᣬ�ټ���2mL���ᣬ���ҡ�ȣ������Թ��м���5mL����Na2CO3��Һ����ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮