��Ŀ����
�����г����и����ӡ�þ���ӡ���������ӵȿ����������⣬��������ɳ�Ȳ��������ʡ�����ʳ�õľ������ô����ᴿ���õ��ġ�ͨ���̲��С����ε��ᴿ�����������ĸ�ʵ��ش��������⡣
��1��ʵ���ҽ���NaCl��Һ����ʱ��һ�������²������� �ٷ��þƾ��ƣ��� �̶���Ȧλ�ã��۷���������(��������ʢ��NaCl��Һ)���ܼ��Ƚ��裻��ֹͣ���ȡ�����ȷ�IJ���˳��Ϊ________________��
��2����ʵ���м���������Լ���˳����ȷ����_____________
A��NaOH BaCl2 Na2CO3
B��BaCl2 Na2CO3�� NaOH
C��BaCl2 NaOH Na2CO3
D��Na2CO3�� NaOH BaCl2
��3��ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ����������ֱ�˵���������������ʹ�ò�������Ŀ�ģ�
�ܽ�ʱ��____________________________________��
����ʱ��____________________________________��
����ʱ��____________________________________��
��ϰ��ϵ�д�
�����Ŀ
4����֪�����£�����Һ�з������·�Ӧ��
��16H++10Z-+2XO4-�T2X2++5Z2+8H2O
��2A2++B2�T2A3++2B-
��2B-+Z2�TB2+2Z-
�ɴ��ƶ�����˵��������ǣ�������
��16H++10Z-+2XO4-�T2X2++5Z2+8H2O
��2A2++B2�T2A3++2B-
��2B-+Z2�TB2+2Z-
�ɴ��ƶ�����˵��������ǣ�������
| A�� | ��ӦZ2+2A2+�T2A3++2Z-���Խ��� | |
| B�� | ZԪ���ڷ�Ӧ���б���ԭ���ڷ�Ӧ���б����� | |
| C�� | ��������ǿ������˳����XO4-��Z2��B2��A3+ | |
| D�� | ��ԭ����ǿ������˳����Z-��B-��A2+��X2+ |
2�� pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������pH����Һ���V�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������pH����Һ���V�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
 pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������pH����Һ���V�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������pH����Һ���V�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | A��B������Һ�����ʵ���Ũ��һ����� | |
| B�� | ϡ�ͺ�A����Һ�����Ա�B����Һǿ | |
| C�� | a=5ʱ��A��ǿ�ᣬB������ | |
| D�� | ��aС��5����A��B�������ᣬ��A����������B |
4��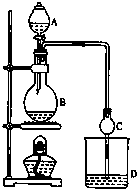 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
��֪��
����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
��ش�
��1��Ũ��������ã���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ���÷�Ӧԭ����д���ܱ�ʾ18O�ڷ�Ӧǰ��λ�ñ仯�Ļ�ѧ����ʽ��CH3COOH+CH3CH218OH CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O��
��2�����θ����C�������Ƿ�ֹ������������
��3����D�з���������������г�����һ�������Ҵ�����ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ�������Ҵ����ټ��루�˿մ�����ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
| �Լ� | �Ҵ� | ���� | �������� |
| �е�/�� | 78.5 | 118 | 77.1 |
��1��Ũ��������ã���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ���÷�Ӧԭ����д���ܱ�ʾ18O�ڷ�Ӧǰ��λ�ñ仯�Ļ�ѧ����ʽ��CH3COOH+CH3CH218OH
 CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O����2�����θ����C�������Ƿ�ֹ������������
��3����D�з���������������г�����һ�������Ҵ�����ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ�������Ҵ����ټ��루�˿մ�����ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�


 N2O3��g����O2��g������N2O3��g��
N2O3��g����O2��g������N2O3��g�� Ϊ
Ϊ