��Ŀ����
16��ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л����б�ͪ��������������������ݸ����ʵ����ʣ����±�����ȷ��ͨ�����в�������Ҵ������ᣮ| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е㣨�棩 | 56.2 | 77.06 | 78 | 117.9 |
�����Һ�м����ռ���Һ��������Һ��pH=10
�ڽ����Һ�����������л�������
���ռ��¶���75��85��ʱ�������
����ȴ����������ƿ�м�Ũ���ᣨ��������Ȼ���ٽ��������������
��ش��������⣺
��1�������ռ�ʹ��Һ��pH=10��Ŀ���ǣ��û�ѧ����ʽ��ʾ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��2����75��85��ʱ��������Ҫ�ɷ����Ҵ������������ƣ���
��3���ڲ�����У��������Ũ�����Ŀ���ǽ�������ת��Ϊ���
��4�������������¶ȿ�����85��125��һ��ʱ�����������Ҫ�ɷ������ᣨ�����ƣ���
���� �����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����ᣬ
��1�������ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룬�����ᷴӦ���������ƣ�������������Ӧ�����Ҵ��������ƣ�
��2���������Ϸ������Ҵ��ķе�78�������
��3���ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������
��4����������ķе�117.9�������
��� �⣺�����ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ����������Ҵ�����Һ����Ҫ����������Һ����ȴ�������м�Ũ���ᣨ����������������ת�������ᣬ�������ռ����
��1�������ռ�ʹ��Һ��pH=10�����Ҵ��е�������������������ת���������ƶ�������룬�����ᷴӦ���������ƣ�������������Ӧ�����Ҵ��������ƣ���Ӧ����ʽΪ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�ʴ�Ϊ��CH3COOH+NaOH��CH3COONa+H2O��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��2���������Ϸ������Ҵ��ķе�78�棬������70��85��ʱ��������Ҫ�ɷ����Ҵ����ʴ�Ϊ���Ҵ���
��3�������Ϸ������ڲ�����У��������Ũ�����Ŀ���ǽ�������ת�������ᣬ����2CH3COONa+H2SO4=Na2SO4+2CH3COOH��
�ʴ�Ϊ����������ת��Ϊ���
��4���������Ϸ���������ķе�117.9�棬��������Ҫ�ɷ������ᣬ�ʴ�Ϊ�����ᣮ
���� ���⿼�����Ʊ���������ƣ���Ŀ�ѶȲ�����ȷ������Ӧԭ��Ϊ���ؼ���ע������������Ӧ��Ũ���ᡢ����̼������Һ��Һ�����ã�����������ѧ���ķ�����������ѧʵ��������

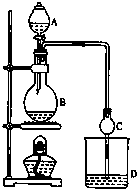 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
| �Լ� | �Ҵ� | ���� | �������� |
| �е�/�� | 78.5 | 118 | 77.1 |
��1��Ũ��������ã���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ���÷�Ӧԭ����д���ܱ�ʾ18O�ڷ�Ӧǰ��λ�ñ仯�Ļ�ѧ����ʽ��CH3COOH+CH3CH218OH
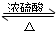 CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O����2�����θ����C�������Ƿ�ֹ������������
��3����D�з���������������г�����һ�������Ҵ�����ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ�������Ҵ����ټ��루�˿մ�����ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g•cm-3�� |
| �Ҵ� | -117.3 | 78.5 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���� | - | 338.0 | 1.84 |
����30mL�Ĵ��Թ�A�а������2��3��3����Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ�ͼ�����Ӻ�װ�ã�װ�����������ã�����С����ȼ���װ�л��Һ�Ĵ��Թ�5��10min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㣮
�ܷ�������������㣬ϴ�ӡ����
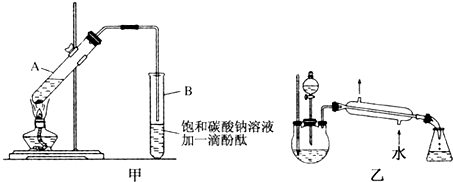
�������ĿҪ��ش��������⣺
��1�����Ƹû��Һ����Ҫ��������Ϊ��һ��30mL�Ĵ��Թ���ע��3mL�Ҵ����ٷֱ�����2mLŨ���ᡢ3mL���ᣨ�Ҵ���Ũ����ļ���˳�ɻ��������ӱ����Թ�ʹ֮��Ͼ��ȣ�
��2�����������ҪС����ȼ��ȣ�����Ҫԭ���Ƿ�Ӧ���Ҵ�������ķе�ϵͣ����ô����ȣ���Ӧ�������������������ʧ�������¶ȹ��߿��ܷ�������ĸ���Ӧ��
��3��ָ����������۲쵽�������Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��
����������������һ���ñ���ʳ��ˮ���Ȼ�����Һϴ�ӣ���ͨ��ϴ����Ҫ��ȥ̼���ƺ��Ҵ��������ƣ����ʣ�Ϊ�˸�������������ѡ�õĸ����ΪB����ѡ����ĸ����
A��P2O5 B����ˮNa2SO4
C����ʯ�� D��NaOH����
��4��ij��ѧ����С���������ͼ����ʾ����ȡ����������װ�ã�ͼ�еIJ���װ����ȥ������ͼ��װ����ȣ�ͼ��װ�õ���Ҫ�ŵ��Т��������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����ķ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ���������������
| A�� | �÷�ӦΪ���淴Ӧ�������ܽ��е��ף����Ҵ���ת����һ���ﲻ��100% | |
| B�� | ����ӷ��������������٣��Ҵ����������ܳ��ת��Ϊ�������� | |
| C�� | ���ﲻ�ȶ����ױ�����Ϊ�������ʶ�Ӱ���Ҵ���ת���� | |
| D�� | ����ﵼ�뱥��̼������ҺҺ���ϣ��н϶�����������ܽ���ˮ��Һ�� |
 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ijѧ����ʵ����������ͼ��ʵ��װ���Ʊ������������о��䷴Ӧ������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ijѧ����ʵ����������ͼ��ʵ��װ���Ʊ������������о��䷴Ӧ������| ���� | �е�/��C | �ܶ�/g?cm-3 |
| �Ҵ� | 78.0 | 0.79 |
| ���� | 117.9 | 1.05 |
| �������� | 77.5 | 0.90 |
| ���촼 | 131 | 0.8123 |
| ���������� | 142 | 0.8670 |
�����Ʒ�Ӧ���Һ��2mLŨH2SO4��3mL�Ҵ���2mL����
�ڰ�ͼ����ʵ��װ�ò�����������
�۷ֱ����Թ�1���Թ�2�м��뷴ӦҺ
���þƾ�����3min���ټ���ʹ֮����3min
�ݷ����ᴿ��������
��1�����Թ�1�м������ʷֱ��ǣ����Ƭ�����ż�����Ⱥ�˳��д�����ƣ����Ҵ�������Ŀ���ǣ���������ת���ʣ�
��2���÷�Ӧ��ƽ�ⳣ������ʽK=$\frac{[C{H}_{3}COOC{H}_{2}C{H}_{3}][{H}_{2}O]}{[CH{\;}_{3}COOH][C{H}_{{\;}_{3}}C{H}_{2}OH]}$��
��3���Թܢ�����ʢ�Լ�Ϊ����̼������Һ�����������ʷ���ʹ�õ�����Ϊ��Һ©�����ڳ���Ĺ����У������ķ�ӦΪ2CH3COOH+Na2CO3�T2CH3COONa+CO2��+H2O��
��4��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ��е��Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� | 1.2 |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
�ڷ���ʵ��A��C����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʣ�Ũ�������ˮ���ܹ���������������ʵ�ԭ����Ũ�����������������Ӧ�����ɵ�ˮ��������������Ũ��ʹƽ�����������������ķ����ƶ���
�ۼ���������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ���Ǵ������ᡢ�Ҵ�δ����Ӧ�����뷴Ӧ��ϵ���¶ȹ��߷���������Ӧ��
��5�������ø�װ���Ʊ����������������ォ��Ҫ��װ���еĵõ�����ô���ʻ�ͣ����ϸߡ���ƫ�͡�����
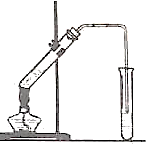 ��ͼ�������Թ����ȼ���2mL95%���Ҵ�������ҡ���»�������3mLŨ���ᣬ�ټ���2mL���ᣬ���ҡ�ȣ������Թ��м���5mL����Na2CO3��Һ����ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮
��ͼ�������Թ����ȼ���2mL95%���Ҵ�������ҡ���»�������3mLŨ���ᣬ�ټ���2mL���ᣬ���ҡ�ȣ������Թ��м���5mL����Na2CO3��Һ����ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮
