��Ŀ����
9�����ʵĽṹ�������ʵ����ʣ���ش������漰���ʽṹ�����ʵ����⣺��1���ڶ������У�Ԫ�صĵ�һ�����ܴ���B��N֮���Ԫ����3�֣�
��2��ijԪ��λ�ڵ������ڢ��壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ�������̬ԭ�ӵļ۲�����Ų�ʽΪ3d84s2��
��3����ϩͪ��CH2=C=O����һ����Ҫ���л��м��壬����CH3COOH�ڣ�C2H5O��3P=O�����¼�����H2O�õ�����ϩͪ������̼ԭ���ӻ����������sp2��sp��1mol��C2H5O��3P=O�����к��еĦҼ�����ĿΪ25NA��
��4����֪��̬NH3��H2O��HF��������ܺͽṹ��ͼ1��
| ������ | ���X-H��Y�� | ����kJ��mol-1 |
| ����HF��n | ��D-H��F | ��28.1 |
| ���� | ��O-H��O | ��18.8 |
| ����NH3��n | ��N-H��N | ��5.4 |

����H2O��HF��NH3�е����ν��͵�ԭ������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n��
��5��̼����Ľṹ����ʯ���ƣ���Ӳ�Ƚ����ڽ��ʯ�����н�ǿ����ĥ���ܣ�̼���辧���ṹ��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����4������̼ԭ�ӵȾ��������̼ԭ����12������֪̼���辧
���߳�Ϊapm����ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ���Ϊ$\frac{\sqrt{11}a}{4}$pm��̼������ܶ�Ϊ$\frac{1.6��1{0}^{32}}{{a}^{3}��{N}_{A}}$g/cm3��
���� ��1��ͬ����Ԫ�صĵ�һ�����ܴ����ҳ��������ƣ�����A�塢VA��ֱ�Ϊȫ�����������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
��2��ijԪ��λ�ڵ������ڢ��壬̼ԭ�ӵĵ����Ų�Ϊ1s22s22p2��δ�ɶԵ�����Ϊ2�����Ԫ��ԭ��δ�Ե�����Ϊ2��
��3����ϩͪ������2��̼ԭ�Ӿ�û�й¶Ե��ӣ�̼ԭ�ӵ��ӻ������ĿΪ3��2������Ϊ�Ҽ���˫������1���Ҽ���1���м���C2H5O��3P=O�����к���25���Ҽ���
��4��ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1�����������Ҫ�˷���������ܼ��ܷ�����
��5�����ݾ�̯�����㾧����Si��Cԭ����Ŀ��ÿ��Siԭ����Χ��4��̼ԭ�ӣ�ԭ����λ����ԭ����Ŀ�ɷ��ȣ����Լ���̼ԭ����Χ�����������Ĺ�ԭ����Ŀ��
�Զ���Cԭ���о�����֮���������Cԭ��λ�������ϣ�ÿ������ԭ��Ϊ8���������ã�ÿ����Ϊ2���������ã�
����1��Siԭ�ӵ���Խ��ߡ�2��̼ԭ�ӵ���Խ��ߣ��ཻ������O�㣬�붥��̼ԭ���γ���ͼ��ʾ��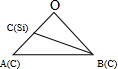 ������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm���������Ҷ�������cos��AOB��ֵ�����������Ҷ�������BC�ij��ȣ�������ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ��룻
������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm���������Ҷ�������cos��AOB��ֵ�����������Ҷ�������BC�ij��ȣ�������ͼ2��1�Ź�ԭ�Ӻ�2��̼ԭ��֮��ľ��룻
��Ͼ�����ԭ����Ŀ����ʾ�������������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺��1��ͬ����Ԫ�صĵ�һ�����ܴ����ҳ��������ƣ��������ڵ�ԭ�ӵ�2p������ڰ����״̬�����ȶ������һ�����ܱ����Ĵ���ԭ�ӵ�2s�������ȫ��״̬����ĵ�һ�����ܱ���Ĵ����Ե�һ�����ܽ�����͵�֮��ĵ�2����Ԫ�����롢̼����3�֣�
�ʴ�Ϊ��3��
��2��ijԪ��λ�ڵ������ڢ��壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ����δ�Ե�����Ϊ2����Ԫ�ؼ۲�����Ų�ʽΪ��3d84s2��
�ʴ�Ϊ��3d84s2��
��3����ϩͪ������2��̼ԭ�Ӿ�û�й¶Ե��ӣ�̼ԭ�ӵ��ӻ������ĿΪ3��2��̼ԭ���ӻ�����Ϊsp2��sp��
����Ϊ�Ҽ���˫������1���Ҽ���1���м���C2H5O��3P=O�����к���25���Ҽ���1mol��C2H5O��3P=O�����к��еĦҼ�����ĿΪ25NA��
�ʴ�Ϊ��sp2��sp��25NA��
��4����������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n����H2O��HF��NH3�е����ν��ͣ�
�ʴ�Ϊ����������ļ����ǣ�HF��n��������NH3��n����ƽ��ÿ�����Ӻ������������2������HF��n�ͣ�NH3��nֻ��1��������Ҫ�˷���������ܼ����DZ�����HF��n����NH3��n��
��5��������Siԭ����ĿΪ4��Cԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��ÿ��Siԭ����Χ��4��̼ԭ�ӣ�ԭ����λ����ԭ����Ŀ�ɷ��ȣ���̼ԭ����λ��Ҳ��4����̼ԭ����Χ�����������Ĺ�ԭ����ĿΪ4��
�Զ���Cԭ���о�����֮���������Cԭ��λ�������ϣ�ÿ������ԭ��Ϊ8���������ã�ÿ����Ϊ2���������ã���̼ԭ�ӵȾ��������̼ԭ����$\frac{8��3}{2}$=12����
����1��Siԭ�ӵ���Խ��ߡ�2��̼ԭ�ӵ���Խ��ߣ��ཻ������O�㣬�붥��̼ԭ���γ���ͼ��ʾ�� ������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm����
������BΪ2��̼ԭ�ӣ�CΪ1��Siԭ�ӣ�1��Siԭ������Χ��4��Cԭ���γ��������壬1��Siԭ���붥��̼ԭ�����ߴ��ھ�����Խ����ϣ��Ҿ���Ϊ��Խ��߳��ȵ�$\frac{1}{4}$����Խ��߳���Ϊ$\sqrt{3}$a pm����OA=OB=$\frac{\sqrt{3}}{2}$a pm����OC=$\frac{\sqrt{3}}{4}$a pm����
��$\frac{\sqrt{3}}{2}$a��2+��$\frac{\sqrt{3}}{2}$a��2-2��$\frac{\sqrt{3}}{2}$a��$\frac{\sqrt{3}}{2}$a��cos��AOB=a2��
���cos��AOB=$\frac{1}{3}$
�ʣ�$\frac{\sqrt{3}}{4}$a��2+��$\frac{\sqrt{3}}{2}$a��2-2��$\frac{\sqrt{3}}{4}$a��$\frac{\sqrt{3}}{2}$a��$\frac{1}{3}$=BC2��
���BC=$\frac{\sqrt{11}a}{4}$
��������Ϊ4��$\frac{28+12}{{N}_{A}}$g�������ܶ�Ϊ4��$\frac{28+12}{{N}_{A}}$g�£�a��10-10 cm��3=$\frac{1.6��1{0}^{32}}{{a}^{3}��{N}_{A}}$g/cm3��
�ʴ�Ϊ��4��12��$\frac{\sqrt{11}a}{4}$��$\frac{1.6��1{0}^{32}}{{a}^{3}��{N}_{A}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ��ӻ��������ѧ�����������������ȣ���4����5��Ϊ�״��㡢�ѵ㣬���ؿ���ѧ�����������������ѶȽϴ�

| ������ | Na+ | Al3+ | Fe3+ | Cu2+ | Ba2+ |
| ������ | OH- | Cl- | CO32- | NO3- | SO42- |
��A��Һ��C��Һ��Ϻ������ɫ��������ó����м�������ϡHNO3�����������ܽ⣬ʣ���ɫ���壻
��B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������C��Һ��D��Һ��Ϻ������ɫ����������C��Һ��D��Һ��Ϻ�������
��B��Һ��D��Һ��Ϻ�������
�ݽ�38.4g CuƬͶ��װ������D��Һ���Թ��У�CuƬ���ܽ⣬�ٵμ�1.6mol•L-1ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�
��1���ݴ��ƶ�A��C�Ļ�ѧʽΪ��ACuSO4��CBa��OH��2��
��2��д��������з�����Ӧ�����ӷ���ʽ2Fe3++3CO32-+3H2O=2Fe��OH��3��+3CO2����
��3��D��Һ�е���ʯ����Һ����������Һ����ɫ��ɺ�ɫ��ԭ����Al3++3H2O?Al��OH��3+3H+�������ӷ���ʽ˵������
��4�����������Ҫ��CuƬ��ȫ�ܽ⣬���ټ���ϡH2SO4�������500mL��

| A�� | a��d��ͬ���칹�� | B�� | b��c��ͬϵ�� | ||
| C�� | ���������Ը��������Һ����a��c | D�� | ֻ��b��c�ܷ���ȡ����Ӧ |

 �������к���C��H��O��N��S��Ԫ�أ�ʳ���е�����Ҫ���������뵰���ʺ������ϳ���������ʽ���ڣ�
�������к���C��H��O��N��S��Ԫ�أ�ʳ���е�����Ҫ���������뵰���ʺ������ϳ���������ʽ���ڣ�
