��Ŀ����
�ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�飮��ش��������⣺
��1������к͵ζ�--�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ��
�����в�����ɲⶨ���ƫ�ߵ��� ����ѡ����ĸ����
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
�ڸ�ѧ����ʵ��������£�
A���ü�ʽ�ζ���ȡϡNaOH 25.00mL��ע����ƿ�У����������ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܣ�
C��������ˮϴ�ɾ��ζ��ܣ�
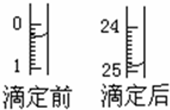
D��ȡ����ʽ�ζ����ñ���HCl��Һ��ϴ����Һע��ζ��̶ܿȡ�0������2��3cm�����ٰѵζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����£�
E�����ζ����Ƿ�©ˮ��
F����ȡ��ƿ�����ظ�����һ�Σ�
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȣ�
a���ζ���������ȷ˳���ǣ��������д�� ��
b����G���������ȷ���յ㣿 ��
c��������̪��ָʾ�������ȷ���յ㣿 ��
��2��������ԭ�ζ�-ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.1mol?L-1�ĸ��������Һ�ζ��������ķ�ӦΪ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+10CO2��+2MnSO4+8H2O�������м�¼��ʵ�����ݣ�
�ٵζ�ʱ��KMnO4��ҺӦװ�� �����ᡱ���ʽ�ζ����У��ζ��յ�ʱ�ζ������� ��
�ڸò�����Һ�����ʵ���Ũ��Ϊ ��
��3�������ζ�--�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܣ�
�ο��±��е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ���� ����ѡ����ĸ����
A��NaCl B��NaBr C��NaCN D��Na2CrO4��
��1������к͵ζ�--�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ��
�����в�����ɲⶨ���ƫ�ߵ���
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
�ڸ�ѧ����ʵ��������£�
A���ü�ʽ�ζ���ȡϡNaOH 25.00mL��ע����ƿ�У����������ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܣ�
C��������ˮϴ�ɾ��ζ��ܣ�
D��ȡ����ʽ�ζ����ñ���HCl��Һ��ϴ����Һע��ζ��̶ܿȡ�0������2��3cm�����ٰѵζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����£�
E�����ζ����Ƿ�©ˮ��
F����ȡ��ƿ�����ظ�����һ�Σ�
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȣ�
a���ζ���������ȷ˳���ǣ��������д��
b����G���������ȷ���յ㣿
c��������̪��ָʾ�������ȷ���յ㣿
��2��������ԭ�ζ�-ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.1mol?L-1�ĸ��������Һ�ζ��������ķ�ӦΪ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+10CO2��+2MnSO4+8H2O�������м�¼��ʵ�����ݣ�
| �ζ����� | ����Һ��� ��mL�� | ��KMnO4��Һ�����mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
�ڸò�����Һ�����ʵ���Ũ��Ϊ
��3�������ζ�--�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܣ�
�ο��±��е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ����
| ������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1.77��10-10 | 5.35��10-13 | 1.21��10-16 | 1.12��10-12 | 1.0��10-12 |
���㣺�к͵ζ�
ר�⣺ʵ����
��������1���ٸ���c�����⣩=
��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��a���ζ�ʵ���м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ��ָʾ�����ζ��Ȳ�����
b�������ü�����ָʾ��ʱ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ����ζ��յ㣻
c�������÷�̪��ָʾ��ʱ�����ν����һ����Һ�ɺ�ɫ����ɫ���Ұ�����ڲ���ɫ����ζ��յ㣻��2���ٸ��ݸ��������Һ����ǿ������ѡ��ζ������ͣ����ݵζ�����ǰ��ҺΪ��ɫ���ζ�����ʱ��Һ����Ϻ�ɫΪ�ζ��յ㣻
�����жϵζ����ݵ���Ч�ԣ�Ȼ���������ı�Һ��ƽ��������ٸ���c�����⣩=
�������������Һ��Ũ�ȣ�
��3���ζ�����ʱ�������μӵζ������ζ�����ָʾ����Ӧ������������ɫ�仯�ij�������֤�ζ����ͱ��ζ�����ȫ��Ӧ��
| c(��)��V(��) |
| V(����) |
��a���ζ�ʵ���м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ��ָʾ�����ζ��Ȳ�����
b�������ü�����ָʾ��ʱ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ����ζ��յ㣻
c�������÷�̪��ָʾ��ʱ�����ν����һ����Һ�ɺ�ɫ����ɫ���Ұ�����ڲ���ɫ����ζ��յ㣻��2���ٸ��ݸ��������Һ����ǿ������ѡ��ζ������ͣ����ݵζ�����ǰ��ҺΪ��ɫ���ζ�����ʱ��Һ����Ϻ�ɫΪ�ζ��յ㣻
�����жϵζ����ݵ���Ч�ԣ�Ȼ���������ı�Һ��ƽ��������ٸ���c�����⣩=
| c(��)��V(��) |
| V(����) |
��3���ζ�����ʱ�������μӵζ������ζ�����ָʾ����Ӧ������������ɫ�仯�ij�������֤�ζ����ͱ��ζ�����ȫ��Ӧ��
���
�⣺��1����A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ���������ĵı�Һ�������ƫС������c�����⣩=
��֪���ⶨ���ƫ�ͣ���A����
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ���˲�����ȷ����Ӱ��ⶨ�������B����
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ�����±�ҺŨ�ȼ�С���ζ�ʱ���ĵı�Һ���������c�����⣩=
��֪���ⶨ���ƫ�ߣ���C��ȷ��
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ���������ĵı�Һ���ƫ����c�����⣩=
��֪���ⶨ���ƫ�ߣ���D��ȷ��
��ѡ��CD��
��a���ζ�ʵ���м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ��ָʾ�����ζ��Ȳ���������˳��ӦΪECBADGF��ECDBAGF���ʴ�Ϊ��ECBADGF��ECDBAGF��
b���ü�����ָʾ��ʱ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ����ζ��յ㣬�ʴ�Ϊ�����ν����һ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ��
c�����÷�̪��ָʾ��ʱ�����ν����һ����Һ�ɺ�ɫ����ɫ���Ұ�����ڲ���ɫ����ζ��յ㣬�ʴ�Ϊ�����ν����һ����Һ�ɺ�ɫ����ɫ���Ұ�����ڲ���ɫ��
��2���ٸ��������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�����Ӧ��ʹ����ʽ�ζ���ʢװ���������Һ���ζ�����ǰ���ҺΪ��ɫ���ζ�����ʱ���Һ������Ϻ�ɫ�����Եζ��յ�����Ϊ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�����εζ����ı�Һ����ֱ�Ϊ����20.40-0.50��mL=19.90mL����23.00-3.00��mL=20.00m����24.10-4.00��mL=20.10mL���ɼ����εζ������ݶ�����Ч�ģ����ı�Һ��ƽ�����Ϊ��
=20.00mL��������ص����ʵ���Ϊ��0.1mol?L-1��0.020L=0.0020mol�����ݷ�Ӧ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O��֪��n��H2C2O4��=
n��KMnO4��=0.005mol�������Һ��������ʵ���Ũ��Ϊ��
=0.2 mol?L-1��
�ʴ�Ϊ��0.2 mol?L-1��
��3������AgNO3ȥ�ζ�NaSCN��Һ����ѡ�õĵζ�ָʾ�������ʵ��ܽ��Ӧ��AgSCN�����������ԣ�ӦΪNa2CrO4�����������ש��ɫ�������ɣ��ʴ�Ϊ��D��
| c(��)��V(��) |
| V(����) |
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ���˲�����ȷ����Ӱ��ⶨ�������B����
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ�����±�ҺŨ�ȼ�С���ζ�ʱ���ĵı�Һ���������c�����⣩=
| c(��)��V(��) |
| V(����) |
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ���������ĵı�Һ���ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
��ѡ��CD��
��a���ζ�ʵ���м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ��ָʾ�����ζ��Ȳ���������˳��ӦΪECBADGF��ECDBAGF���ʴ�Ϊ��ECBADGF��ECDBAGF��
b���ü�����ָʾ��ʱ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ����ζ��յ㣬�ʴ�Ϊ�����ν����һ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ��
c�����÷�̪��ָʾ��ʱ�����ν����һ����Һ�ɺ�ɫ����ɫ���Ұ�����ڲ���ɫ����ζ��յ㣬�ʴ�Ϊ�����ν����һ����Һ�ɺ�ɫ����ɫ���Ұ�����ڲ���ɫ��
��2���ٸ��������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�����Ӧ��ʹ����ʽ�ζ���ʢװ���������Һ���ζ�����ǰ���ҺΪ��ɫ���ζ�����ʱ���Һ������Ϻ�ɫ�����Եζ��յ�����Ϊ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�����εζ����ı�Һ����ֱ�Ϊ����20.40-0.50��mL=19.90mL����23.00-3.00��mL=20.00m����24.10-4.00��mL=20.10mL���ɼ����εζ������ݶ�����Ч�ģ����ı�Һ��ƽ�����Ϊ��
| 19.90mL+20.00mL+20.10mL |
| 3 |
| 5 |
| 2 |
| 0.005mol |
| 0.025L |
�ʴ�Ϊ��0.2 mol?L-1��
��3������AgNO3ȥ�ζ�NaSCN��Һ����ѡ�õĵζ�ָʾ�������ʵ��ܽ��Ӧ��AgSCN�����������ԣ�ӦΪNa2CrO4�����������ש��ɫ�������ɣ��ʴ�Ϊ��D��
���������⿼��������к͵ζ�������ע�������к͵ζ��IJ���������ָʾ����ѡ�����������������е��Ѷȵ����⣬ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ���������

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ
ѹǿ�����з�Ӧ��ϵ�ķ�Ӧ����Ӱ�첻����ǣ�������
| A��N2+3H2?2NH3 |
| B��H2 ��g��+I2 ��g��?2HI��g�� |
| C��2NO2?N2O4 |
| D��HCl+NaOH?NaCl+H2O |
ij����NaOH�������˿����е�CO2���������ʣ���Ҫ���ù������Ƴɽϴ�����Һ��������Ҫ��ʵ���������Ӧ�ǣ�������
| A���ܽ��������BaCl2��Һ������ |
| B���ܽ��������CaCl2��Һ������ |
| C���ܽ��������Ca��OH��2��Һ������ |
| D���ܽ����������������� |
�ڼס��ҡ�������4ֻ����ƿ�зֱ�װ��Cl2��H2��HCl��HBr�е�һ�����壬�����Ķ���ȼ�ܹ��ڼ��а���ȼ�գ��ס�����ƿ��Ϻ�ƿ���Ͽɼ�����ɫСҺ�Σ�����ƿ�е������ǣ�������
| A��HBr |
| B��HCl |
| C��H2 |
| D��Cl2 |
���и��������У��������ͼʾ������ͨ��������һ��ת����ϵ�����ֻ�У�������
| ��� | X | Y | Z | W |  |
| �� | Cu | CuSO4 | Cu��OH��2 | CuO | |
| �� | Na | NaOH | Na2CO3 | NaCl | |
| �� | Cl2 | Ca��ClO��2 | HClO | HCl | |
| �� | Fe | FeCl3 | FeCl2 | Fe��OH��2 |
| A���٢ڢ� | B���٢ۢ� |
| C���ڢ� | D���٢� |
һ���¶��£���aL�ܱ������м���2molNO2��g�����������·�Ӧ��2NO2?2NO+O2���˷�Ӧ�ﵽƽ��״̬ʱ�ı�־�ǣ�������
| A������������ɫ��dz |
| B�������������ʵ������ |
| C���������NO2��NO��O2���ʵ���֮��Ϊ2��2��1 |
| D����λʱ��������2nmolNOͬʱ����2nmolNO2 |


 ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�
ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�