��Ŀ����
�ڡ����ݸ�Ŀ���мǵ���һ����һ�����ص�ҩ��-�����㣬�������к���һ�����صĻ�ɫ����-�����أ���������ӡ�ȱ���������Ȼ����������Ϊ��ӡ�ȵĹ���ƽ𡱣������غϳ�·�����£�

��֪��R1-CHO+
 +H2O��R1��R2��R3Ϊ��������ԭ�ӣ�
+H2O��R1��R2��R3Ϊ��������ԭ�ӣ�
��1����д���ٵķ�Ӧ������ ���ܵķ�Ӧ������ ��
��2��Ҫ���� �к��з��ǻ����õ��Լ��� ��
�к��з��ǻ����õ��Լ��� ��
��3���ֱ�д�ڡ��ۡ��ݳ���Ӧ�Ļ�ѧ����ʽ��
�� ���� ���� ��

��֪��R1-CHO+

| NaOH(aq) |
| �� |
 +H2O��R1��R2��R3Ϊ��������ԭ�ӣ�
+H2O��R1��R2��R3Ϊ��������ԭ�ӣ���1����д���ٵķ�Ӧ������
��2��Ҫ����
 �к��з��ǻ����õ��Լ���
�к��з��ǻ����õ��Լ�����3���ֱ�д�ڡ��ۡ��ݳ���Ӧ�Ļ�ѧ����ʽ��
��
���㣺�л���ĺϳ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������ϩ���巢���ӳɷ�Ӧ���ɢ�ΪBrCH2CH2Br���ɷ�Ӧ�ܿ�֪��ΪOHC-CHO����ΪHOCH2CH2OH���������Ϣ��֪��Ϊ ���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ��
���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ�� ���Դ˽����⣮
���Դ˽����⣮
 ���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ��
���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ�� ���Դ˽����⣮
���Դ˽����⣮���
�⣺��ϩ���巢���ӳɷ�Ӧ���ɢ�ΪBrCH2CH2Br���ɷ�Ӧ�ܿ�֪��ΪOHC-CHO����ΪHOCH2CH2OH���������Ϣ��֪��Ϊ ���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ��
���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ�� ��
��
��1����Ϊ��ϩ�ļӳɷ�Ӧ����Ϊȩ��������Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��2�� ���з��ǻ�������FeCl3��Һ��Ӧ����Һ����ɫ���ʴ�Ϊ��FeCl3��Һ��
���з��ǻ�������FeCl3��Һ��Ӧ����Һ����ɫ���ʴ�Ϊ��FeCl3��Һ��
��3����ΪBrCH2CH2Br��ˮ�ⷴӦ������ʽΪBrCH2CH2Br+2NaOH
HOCH2CH2OH+2NaBr����ΪHOCH2-CH2OH��������Ӧ������ʽΪHOCH2-CH2OH+O2
OHC-CHO+2H2O���ݷ�Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ��BrCH2CH2Br+2NaOH
HOCH2CH2OH+2NaBr��HOCH2-CH2OH+O2
OHC-CHO+2H2O�� ��
��
 ���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ��
���������ȩ�Ľṹ��ʽ��֪�����صĽṹ��ʽΪ�� ��
����1����Ϊ��ϩ�ļӳɷ�Ӧ����Ϊȩ��������Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��2��
 ���з��ǻ�������FeCl3��Һ��Ӧ����Һ����ɫ���ʴ�Ϊ��FeCl3��Һ��
���з��ǻ�������FeCl3��Һ��Ӧ����Һ����ɫ���ʴ�Ϊ��FeCl3��Һ����3����ΪBrCH2CH2Br��ˮ�ⷴӦ������ʽΪBrCH2CH2Br+2NaOH
| ˮ |
| �� |
| Cu |
| �� |
 ��
���ʴ�Ϊ��BrCH2CH2Br+2NaOH
| ˮ |
| �� |
| Cu |
| �� |
 ��
��
���������⿼���л����ƶϣ���Ҫѧ���Ը������Ϣ�������ã��ۺϷ���ȷ��E�Ľṹ�ǹؼ�����ϵĿ���ѧ����ѧ�������������������ע�����չ�������������ת�����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
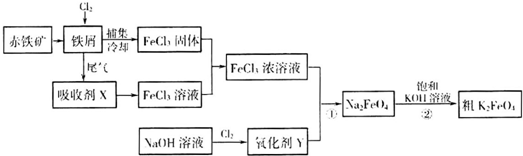
 ��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN-���ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN-����������OCN-���������������¼�����NaClO������N2��CO2��
��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN-���ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN-����������OCN-���������������¼�����NaClO������N2��CO2��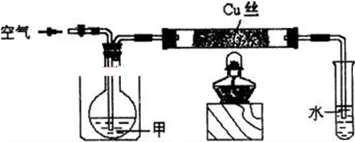



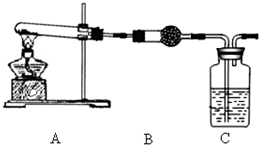
 ��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��
��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��