��Ŀ����
��ͼ�У�����C��H��O����Ԫ����ɣ�Ԫ�ص�������Ϊ12��3��8���ķе�Ϊ78.5�棬��������H2������ܶ���23�����¶ȿ�����400�����£���Ҫ�����ʵ�飮
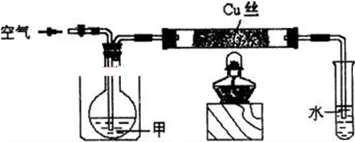
��1����ͨ������������½���ʵ���
�ټ������� ������ҩƷ��IJ��������� ��
a������������b���ù������Ϲ������������ c����ȼ�ƾ��Ƹ�ͭ˿����
��ʵ�������ȡ�Թ��е���Һ����Cu��OH��2��ϣ����������ڣ�ʵ������Ϊ ��
��2����ֹͣͨ������������½���ʵ���
�ٹرջ�����Ϊʹ�׳������뷴Ӧ���У���Ҫ���еIJ����� ��
�ڼ��������뷴Ӧ�ܺ���ͭ��������250��350�������·�������Ļ�ѧ��Ӧ�����Թ����ռ�����ʵ�����ͬ�IJ�����п�ȼ�����嵥�ʷų����÷�Ӧ��ʾ�˼״������ı��ʣ�д��ʵ����з�Ӧ�Ļ�ѧ����ʽ������ϸû�ѧ����ʽ��Ҫ˵��ʵ�������ͨ���������ã���ƽ���ƶ����۽��ͣ��� �� ��

��1����ͨ������������½���ʵ���
�ټ�������
a������������b���ù������Ϲ������������ c����ȼ�ƾ��Ƹ�ͭ˿����
��ʵ�������ȡ�Թ��е���Һ����Cu��OH��2��ϣ����������ڣ�ʵ������Ϊ
��2����ֹͣͨ������������½���ʵ���
�ٹرջ�����Ϊʹ�׳������뷴Ӧ���У���Ҫ���еIJ�����
�ڼ��������뷴Ӧ�ܺ���ͭ��������250��350�������·�������Ļ�ѧ��Ӧ�����Թ����ռ�����ʵ�����ͬ�IJ�����п�ȼ�����嵥�ʷų����÷�Ӧ��ʾ�˼״������ı��ʣ�д��ʵ����з�Ӧ�Ļ�ѧ����ʽ������ϸû�ѧ����ʽ��Ҫ˵��ʵ�������ͨ���������ã���ƽ���ƶ����۽��ͣ���
���㣺�л�����ƶ�
ר�⣺
��������1������C��H��O����Ԫ����ɣ�Ԫ�ص�������Ϊ12��3��8����N��C����N��H����N��O��=
��
��
=2��6��1�������ʽΪC2H6O����������H2������ܶ���23����֪��Է�������Ϊ46����ķ���ʽΪC2H6O������ͭ˿��������������Ӧ��ӦΪ�Ҵ�����ͭΪ���������¼��ȣ�����������������ԭ��Ӧ������ȩ��ӦΪ2CH3CH2OH+O2
2CH3CHO+2H2O����ȩ��������������ͭ��Һ�ڼ��������·���������ԭ��Ӧ���������������ͭ����Ӧ�ķ���ʽΪCH3CHO+2Cu��OH��2
CH3COOH+Cu2O+2H2O��
��2������ˮԡ���ȵķ���ʹ�Ҵ��ӷ����ṩ�Ҵ����壬�ɢ���������Ҵ���ͭ������������������ȩ����������ƽ���ƶ��ĽǶȷ���ͨ����������ã�
| 12 |
| 12 |
| 3 |
| 1 |
| 8 |
| 16 |
| ���� |
| �� |
| �� |
��2������ˮԡ���ȵķ���ʹ�Ҵ��ӷ����ṩ�Ҵ����壬�ɢ���������Ҵ���ͭ������������������ȩ����������ƽ���ƶ��ĽǶȷ���ͨ����������ã�
���
�⣺��1���ټ���C��H��O����Ԫ����ɣ�Ԫ�ص�������Ϊ12��3��8����N��C����N��H����N��O��=
��
��
=2��6��1�������ʽΪC2H6O����������H2������ܶ���23����֪��Է�������Ϊ46����ķ���ʽΪC2H6O������ͭ˿��������������Ӧ��ӦΪ�Ҵ�������ͭ˿��������������Ӧ��ӦΪ�Ҵ�������ҩƷ���ȵ�ȼ�ƾ��Ƹ�ͭ˿���ȣ�Ȼ�����������ù������Ϲ���������ʴ�Ϊ���Ҵ���cab��
��ʵ�������ȡ�Թ��е���Һ�����Ƶ�Cu��OH��2��ϣ����������ڣ���ȩ��������������ͭ��Һ�ڼ��������·���������ԭ��Ӧ���������ש��ɫ��������ͭ����Ӧ�ķ���ʽΪCH3CHO+2Cu��OH��2
CH3COOH+Cu2O+2H2O��
�ʴ�Ϊ����ש��ɫ�������ɣ�
��2���ٿ���ˮԡ���ȵķ���ʹ�Ҵ��ӷ����ṩ�Ҵ����壬ˮԡ����ʱ���¶�Ӧ��78.5�����ϣ���ʹ�Ҵ��ӷ���
�ʴ�Ϊ�����ձ��м���ˮ����78.5�������¶ȼ��ȣ�
���Ҵ���ͭ������������������ȩ����������Ӧ�ķ���ʽΪCH3CH2OH
CH3CHO+H2����ӦΪ���淴Ӧ����֪ʵ�������ͨ������������ʹ�����������������ˮ��ʹƽ��������Ӧ�����ƶ���
�ʴ�Ϊ��CH3CH2OH
CH3CHO+H2��ʹ�����������������ˮ��ʹƽ��������Ӧ�����ƶ���
| 12 |
| 12 |
| 3 |
| 1 |
| 8 |
| 16 |
��ʵ�������ȡ�Թ��е���Һ�����Ƶ�Cu��OH��2��ϣ����������ڣ���ȩ��������������ͭ��Һ�ڼ��������·���������ԭ��Ӧ���������ש��ɫ��������ͭ����Ӧ�ķ���ʽΪCH3CHO+2Cu��OH��2
| �� |
�ʴ�Ϊ����ש��ɫ�������ɣ�
��2���ٿ���ˮԡ���ȵķ���ʹ�Ҵ��ӷ����ṩ�Ҵ����壬ˮԡ����ʱ���¶�Ӧ��78.5�����ϣ���ʹ�Ҵ��ӷ���
�ʴ�Ϊ�����ձ��м���ˮ����78.5�������¶ȼ��ȣ�
���Ҵ���ͭ������������������ȩ����������Ӧ�ķ���ʽΪCH3CH2OH
| 250-350�� |
| Cu |
�ʴ�Ϊ��CH3CH2OH
| 250-350�� |
| Cu |
�����������ۺ��л��ﻯѧ��Ӧԭ����̽����������ѧ���ļ�������������������ʵ������������������Ϊ�߿��������ͣ��ѶȽϴ�ע����������Ϣ��Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�����������ڴ�������ǣ�������
| A��Ư�� | B������ | C����ˮ | D��Һ�� |
�����и�����������Һ�У�һ���ܴ���������������ǣ�������
| A����ˮ�����c��H+��=l��l0-14mol/L����Һ�У�Ca2+��K+��Cl-��HCO3- |
| B����ʹpH��ֽ�ʺ�ɫ����Һ��Na+��NH4+��I-��NO3- |
| C��Kw/c��H+��=0.1mol/L����Һ��Na+��K+��SiO32-��NO3- |
| D��FeCl2��Һ��K+��Na+��SO42-��AlO2- |
 ��1��a��b��c�����д���ĵ���̶�������
��1��a��b��c�����д���ĵ���̶�������


 +H2O��R1��R2��R3Ϊ��������ԭ�ӣ�
+H2O��R1��R2��R3Ϊ��������ԭ�ӣ� �к��з��ǻ����õ��Լ���
�к��з��ǻ����õ��Լ���