��Ŀ����
9��ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3%����1��A�ķ���ʽΪC8H8��
��2��A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽΪ
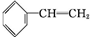 +Br2
+Br2
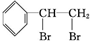 ��
����3����֪
 $\stackrel{ϡ����KMnO_{4}����Һ/OH-}{��}$
$\stackrel{ϡ����KMnO_{4}����Һ/OH-}{��}$ ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ ��
����4��һ�������£�A��������Ӧ���õ��Ļ�������̼����������Ϊ85.7%��д���˻�����Ľṹ��ʽ
 ��
����5����һ�������£���A�ۺϵõ��ĸ߷��ӻ�����Ľṹ��ʽΪ
 ��
��
���� ��1��������Է��������ͺ�̼���ɼ��㺬��������������C��Hԭ����Ŀ����֪����ʽ��
��2�������ʺ��б����������巢����Ӧ��˵��AӦΪ����ϩ��
��3��A�к���C=C�������Ϣ��֪�����
��4��һ�������£�A��������Ӧ���ɵ��ұ����һ������飬��Ϻ������жϣ�
��5������C=C���ɷ����Ӿ۷�Ӧ���ɾ۱���ϩ��
��� �⣺��1��1molA��n��C��=$\frac{104g��92.3%}{12g/mol}$=8mol��n��H��=$\frac{104-12��8}{1}$=8����A�ķ���ʽΪ��C8H8��
�ʴ�Ϊ��C8H8��
��2�������ʺ��б����������巢����Ӧ��˵��AӦΪ����ϩ���ṹΪ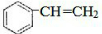 �����巢���ӳɷ�Ӧ����
�����巢���ӳɷ�Ӧ���� ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ +Br2
+Br2
 ��
��
�ʴ�Ϊ�� +Br2
+Br2
 ���ӳɷ�Ӧ��
���ӳɷ�Ӧ��
��3���γ��ڶ����ṹ���ô���ϩ����������ԭ�ӣ���֪ ����
���� ����Ӧ�ķ���ʽΪ��
����Ӧ�ķ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4��һ�������£�A��������Ӧ���ɵ��ұ����һ������飬�õ��Ļ�������̼����������Ϊ85.7%��ӦΪ �����Цأ�C��=$\frac{12��8}{12��8+16}$=85.7%��
�����Цأ�C��=$\frac{12��8}{12��8+16}$=85.7%��
�ʴ�Ϊ�� ��
��
��5��A�����к���C=C���ɷ����Ӿ۷�Ӧ���ɾ۱���ϩ����ṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬��Ŀ�Ѷ��еȣ�ע�����Ԫ�صĺ�������Է��������ƶ�AΪ������Ĺؼ���ѧϰ��ע���л�������ŵ����ʣ�


| A�� | ԭ��ص��ܷ�ӦΪ Fe+Cu2+=Fe2++Cu | |
| B�� | ��Ӧǰ���缫������ȣ�һ��ʱ������缫�������12g��������ͨ��0.2 mol���� | |
| C�� | �����������䣬����CuCl2��Һ��ΪNH4Cl��Һ��ʯī�缫��ӦʽΪ2H++2e-=H2�� | |
| D�� | ��������KNO3��Һ����������NO3-�������ձ� |
| A�� | ��״���£�46 g�Ҵ��к��еĹ��ۼ�����Ϊ8 NA | |
| B�� | �ں�Al3+����ΪNA��AlCl3��Һ�У�Cl-����Ϊ3NA | |
| C�� | ���³�ѹ�£�7.8 gNa2S��Na2O2�Ļ�����У�����������������Ϊ1.8 NA | |
| D�� | һ�������£�2molSO2��1molO2������ܱ������г�ַ�Ӧ�������еķ���������2NA |
| A�� | �縺�Խϴ��Ԫ���ڻ�������ֻ���γɸ��ۣ������γ����� | |
| B�� | ��Ԫ��X�ĵ�һ�����ܱ�Ԫ��Y�ĵ�һ������С����X�Ľ�����Բ�һ��ǿ��Y | |
| C�� | HԪ����FԪ�صĵ縺�Բ�ֵ��1.9������HF�������ӻ����� | |
| D�� | ijԪ��ԭ����Χ�����Ų�ʽΪ3d64s2�����Ԫ�ؿ�������+2��+3�ۣ���+3���ȶ� |

| A�� | �����õ���ˮ�������X�Լ�����Һ1��Ӧ�ƽ���1 | |
| B�� | Y�Լ���ͨ���ȼҵ�Ƶ� | |
| C�� | ����Z����Һ2�ĵ�һ����Ӧ�ǣ�2OH-+CO2�TCO32-+H2O | |
| D�� | ��ҵ���õ�����4����ȡ����5���������ʯ�������ǽ�����4���۵� |
 NCl3��һ�ֻ�ɫճ������״Һ�壨��֪�縺�ԣ�N��Cl��װ����ͼ��ʾ�����ö��Ե缫����Ʊ���ԭ���ǣ�NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2��������˵��������ǣ�������
NCl3��һ�ֻ�ɫճ������״Һ�壨��֪�縺�ԣ�N��Cl��װ����ͼ��ʾ�����ö��Ե缫����Ʊ���ԭ���ǣ�NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2��������˵��������ǣ�������| A�� | b�缫�ӵ�Դ�ĸ��� | |
| B�� | ��������������Ԫ��ΪCl | |
| C�� | ����ҺX�в���HCl | |
| D�� | ÿ����3molH2����6molCl-ͨ�������ӽ���Ĥ |
| A�� | 2��7 | B�� | 7��15 | C�� | 3��8 | D�� | 1��5 |
 ��
�� ��д��A��B��Ԫ�ذ�1��1ԭ�Ӹ������γɻ�����ĵ���ʽ
��д��A��B��Ԫ�ذ�1��1ԭ�Ӹ������γɻ�����ĵ���ʽ ��
��
 ��
��