��Ŀ����
11���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�飮��ش��������⣺��1������к͵ζ�--�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ��
�����в�������ɲⶨ���ƫ�ߵ���CD����ѡ����ĸ����
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ
B��ʢNaOH��Һ��ƿ������ˮϴ��δ��NaOH��Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
��ijѧ����ʵ��������£�
A���ü�ʽ�ζ���ȡϡNaOH��Һ25.00mL��ע����ƿ�У����������ָ��
B���ô��ⶨ����Һ��ϴ��ʽ�ζ���
C��������ˮϴ�ɾ��ζ���
D��ȡ����ʽ�ζ��ܣ��ñ�HCl��Һ��ϴ��ע���Һ����0���̶��� ��2��3cm�����ٰѵζ��̶ܹ��ã�����Һ������0���̶Ȼ�0���̶�����
E�����ζ����Ƿ�©ˮ
F����ȡ��ƿ�����ظ�����һ��
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶�
a���ζ���������ȷ˳���ǣ��������д��ECBADGF��
b����G���������ȷ���ζ��յ㣿���ν����һ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ��
��2��������ԭ�ζ���ȡ������Һ����ƿ�У�������ϡ���ᣬ��Ũ��Ϊ0.1mol•L-1�ĸ��������Һ�ζ���������ӦΪ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+10CO2��+22MnSO4+8H2O�������¼��ʵ�����ݣ�
| �ζ����� | ����Һ��� ��mL�� | ��KMnO4��Һ�����mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
�ڸò�����Һ�����ʵ���Ũ��Ϊ0.2mol/L��
���� ��1���ٸ���c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������c��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��a�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�����
b�������ü�����ָʾ��ʱ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ����ζ��յ㣻
��2���ٸ��ݸ��������Һ����ǿ������ѡ��ζ������ͣ����ݵζ�����ǰ��ҺΪ��ɫ���ζ�����ʱ��Һ����Ϻ�ɫƫ��ζ��յ㣻
�����жϵζ����ݵ���Ч�ԣ�Ȼ���������ı�Һ��ƽ��������ٸ���c�����⣩=$\frac{c��������V������}{V�����⣩}$���������Һ��Ũ�ȣ�
��� �⣺��1����A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ���������ĵı�Һ�������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ͣ���A����
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ���˲�����ȷ����Ӱ��ⶨ�������B����
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ�����±�ҺŨ�ȼ�С���ζ�ʱ���ĵı�Һ���������c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ߣ���C��ȷ��
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ���������ĵı�Һ���ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨ���ƫ�ߣ���D��ȷ��
�ʴ�Ϊ��CD��
��a���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ��������Եζ���������ȷ˳����ECBADGF��
�ʴ�Ϊ��ECBADGF��
b���ü�����ָʾ��ʱ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ����ζ��յ㣬
�ʴ�Ϊ�����ν����һ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ��
��2���ٸ��������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�����Ӧ��ʹ����ʽ�ζ���ʢװ���������Һ���ζ�����ǰ���ҺΪ��ɫ���ζ�����ʱ���Һ������Ϻ�ɫ�����Եζ��յ�����Ϊ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ڵ�һ�εζ����ı�Һ���Ϊ����20.40-0.50��mL=19.90mL��
�ڶ��εζ����ı�Һ���Ϊ����23.00-3.00��mL=20.00mL��
�����εζ����ı�Һ���Ϊ����24.10-4.00��mL=20.10mL��
�ɼ����εζ������ݶ�����Ч�ģ����ı�Һ��ƽ�����Ϊ��$\frac{19.90mL+20.00mL+20.10mL}{3}$=20.00mL��������ص����ʵ���Ϊ��0.10mol/L��0.020L=0.0020mol�����ݷ�Ӧ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O��֪��n��H2C2O4��=$\frac{5}{2}$n��KMnO4��=0.005mol��
����Һ��������ʵ���Ũ��Ϊ$\frac{0.005mol}{0.025L}$=0.2 mol•L-1��
�ʴ�Ϊ��0.2 mol•L-1��
���� ���⿼��������к͵ζ���������ԭ�ζ���ע�����յζ��IJ��������������������ͼ��ɣ����������ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���Al ��Al2O3��Al��OH��3 ��NaHSO3�ݣ�NH4��2CO3�ޣ�CH3COO��2Cu��NH2-CH2-COOH��
| A�� | �٢ڢۢ� | B�� | ���ݢ��� | C�� | �ڢۢ� | D�� | ȫ�� |
| A�� | 25��ʱ��FeS��ZnS��CuS���ܽ���������� | |
| B�� | ZnS������Һ�м�������NazS���壬ƽ�����Һ��c��Zn2+��•c��S2-��=Ksp��ZnS����c��Zn2+��=c��S2-�� | |
| C�� | ��ȥ��ҵ��ˮ�е�Cu2+������FeS��Ϊ������ | |
| D�� | ij��Һ�к���Fe2+��Cu2+��Znz+��Ũ�Ⱦ�Ϊ0.010 moI•L-1�������Һ����μ���0.010 mol•L-1��Na2S��Һʱ��Fe2+���ȳ������� |
| A�� | ��ʽ�ζ�����װ��Һǰδ�ñ�������Һ��ϴ2��3�� | |
| B�� | ��ʼʵ��ʱ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ�������������ʧ | |
| C�� | ʢNaOH��Һ����ƿ�ζ�ǰ��NaOH��Һ��ϴ2��3�� | |
| D�� | ��ƿ����Һ��ɫ�ɻ�ɫ���ɫ���������µζ�����Һ�����ڿ̶� |
| �۵�/�� | �е�/�� | |
| 1-���� | -89.53 | 117.25 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
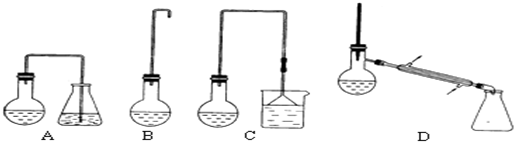
��1���Ʊ�1-�嶡���װ��Ӧѡ����ͼ�е�C������ţ�����Ӧ����ʱ���¶Ȳ��˳���100�棬�����Ƿ�ֹ1-�嶡�����������ݳ���Ӱ��������¶�̫�ߣ�Ũ������������ǿ�����������廯�⣮
��2���Ʊ������У������Ũ������廯�Ƶ������Ƕ��߷�Ӧ����HBr��
��3����Ӧ��������Ӧ�������1-�嶡����������Ӧѡ�õ�װ����D��������ţ����ò���Ӧ���Ƶ��¶ȣ�t����Χ��101.6���t��117.25�森
��4������ȥ������е���������Br2���������������ʺϵ���c��������ĸ��
a��NaI b��NaOH c��Na2SO3 d��KCl��
 ����Ϊ2��3-����-2-��ϩ
����Ϊ2��3-����-2-��ϩ
 ��
��