��Ŀ����
9��ij��A����Է�������Ϊ84���ش��������⣺��1����A�ķ���ʽΪC6H12
��2������������A�����������ϣ��������ʵ���һ�������ȼ���������������������A����������һ�������ȼ���������������������C��
A�� C7H8 B��C6H14 C��C7H14 D��C8H8
��3������AΪ���������������е�̼ԭ����ͬһƽ���ϣ��÷��ӵ�һ��ȡ����ֻ��һ�֣�
��A�Ľṹ��ʽΪ
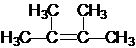 ����Ϊ2��3-����-2-��ϩ
����Ϊ2��3-����-2-��ϩ������A��Br2��CCl4��Һ��Ӧ����B��д����A����B�Ļ�ѧ����ʽ��Br2+
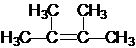 ��
��
����A��Ϊͬ���칹�壬������̼ԭ�Ӹ�����A��ͬ��������3�֣�������A��
��4����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���A�Ľṹ��ʽΪ
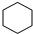 ��
��
���� ��1��ij��A����Է�������Ϊ84��������Cԭ�������Ŀ=$\frac{84}{12}$=7�����л������ʽΪC6H12��
��2������ͨʽΪCxHy���������ʵ���һ����������ֺ�������x+$\frac{y}{4}$����ȣ�����������������ȣ���������һ�������ȼ�����������������䣬�����������ʽ��ͬ��
��3��������AΪ������Ӧ����һ��̼̼˫�������������е�̼ԭ����ͬһƽ���ϣ��÷��ӵ�һ��ȡ����ֻ��һ�֣�ֻ��1����ԭ�ӣ�
��AΪϩ����1molA��1mol�����巢���ӳɷ�Ӧ���ɶ�����
����A��Ϊͬ���칹�壬������̼ԭ�Ӹ�����A��ͬ�������У�CH2=C��CH2��CH��CH3��2��CH2=CHC��CH3��3��CH2=C��CH2CH3��2��
��4��A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���C=C��ֻ��һ��H��ӦΪ����
��� �⣺��1��ij��A����Է�������Ϊ84��������Cԭ�������Ŀ=$\frac{84}{12}$=7����Ϊ���л�����HԪ�أ�����Cԭ����ĿΪ6����Hԭ����Ϊ$\frac{84-12��6}{1}$=12�����л������ʽΪC6H12��
�ʴ�Ϊ��C6H12��
��2������ͨʽΪCxHy���������ʵ���һ����������ֺ�������x+$\frac{y}{4}$����ȣ�����������������ȣ�������ȣ�C6H12�ĺ�����Ϊ9��C7H8 �ĺ�����Ϊ9�����Զ���
�����ʵ���һ�������ȼ�����������������䣻
��������һ�������ȼ�����������������䣬�����������ʽ��ͬ��C6H12�����ʽΪCH2��C7H14�����ʽΪCH2��
�ʴ�Ϊ��A��C��
��3��������AΪ������Ӧ����һ��̼̼˫�������������е�̼ԭ����ͬһƽ���ϣ��÷��ӵ�һ��ȡ����ֻ��һ�֣���AΪ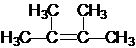
�ʴ�Ϊ��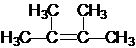
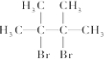
��AΪϩ����1molA��1mol�����巢���ӳɷ�Ӧ���ɶ������䷴Ӧ����ʽΪ��Br2+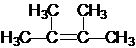
 ��
��
�ʴ�Ϊ��Br2+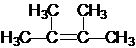
 ��
��
����A��Ϊͬ���칹�壬������̼ԭ�Ӹ�����A��ͬ�������У�CH2=C��CH2��CH��CH3��2��CH2=CHC��CH3��3��CH2=C��CH2CH3��2����A��ͬ���칹����ĿΪ3��
�ʴ�Ϊ��3��
��4����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���AΪ�����飬�ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϡ��л���ȼ�պ��������⡢��������ͬ���칹����д�ȣ���Ŀ�Ƚ��ۺϣ��ǶԻ���֪ʶ���ۺϿ��飬�Ѷ��еȣ�

 ��ǰ����ϵ�д�
��ǰ����ϵ�д���̫������ֽ�ˮ���⣺2H2O��l���T2H2��g��+O2��g����H1=+571.6kJ•mol-1
�ڽ�̿��ˮ��Ӧ���⣺C��s��+H2O��g���TCO��g��+H2��g����H2=+131.3kJ•mol-1
�ۼ�����ˮ��Ӧ���⣺CH4��g��+H2O��g���TCO��g��+3H2��g����H3=+206.1kJ•mol-1��
| A�� | ��Ӧ���е���ת��Ϊ��ѧ�� | |
| B�� | ��Ӧ��Ϊ���ȷ�Ӧ | |
| C�� | ��Ӧ��ʹ�ô�������H3��С | |
| D�� | ��ӦCH4��g��=C��s��+2H2��g���ġ�H=+74.8 kJ•mol-1 |
| A�� | Na+��Mg2+��CO32-��I- | B�� | H+��NO3-��Fe2+��Br- | ||
| C�� | NH4+��K+��SO42-��Cl- | D�� | Na+��S2-��Cu2+��SO32- |
| A�� | ��������Na2CO3���壬ƽ������ƣ�ƽ������ƣ���Һ��c��F-������ | |
| B�� | ��������NaOH������c��F-����c��HF������Һһ���ʼ��� | |
| C�� | ��������Na��pH=8����C��Na+��-c��F-��=0.99��10-7mol•L-1 | |
| D�� | �����������ᣬ$\frac{c��{F}^{-}��}{c��HF��•c��O{H}^{-}��}$���� |

| �������� | ʵ������ | ����ԭ�� |
| ��K1���ƶ�ע����������ʹX�е����建��ͨ��Y���� | ��Y���к���ɫ����������dz | �ڷ�Ӧ�Ļ�ѧ����ʽ 8NH3+6NO2 $\frac{\underline{\;����\;}}{\;}$7N2+12H2O |
| ��ע���������˻�ԭ�����̶�����װ�ûָ������� | Y����������ˮ�� | ���ɵ���̬ˮ���� |
| ��K2 | ��Z��NaOH��Һ������������ | �ܷ�Ӧ��������������٣�Y����ѹǿС����ѹ |
��1������к͵ζ�--�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ��
�����в�������ɲⶨ���ƫ�ߵ���CD����ѡ����ĸ����
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ
B��ʢNaOH��Һ��ƿ������ˮϴ��δ��NaOH��Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
��ijѧ����ʵ��������£�
A���ü�ʽ�ζ���ȡϡNaOH��Һ25.00mL��ע����ƿ�У����������ָ��
B���ô��ⶨ����Һ��ϴ��ʽ�ζ���
C��������ˮϴ�ɾ��ζ���
D��ȡ����ʽ�ζ��ܣ��ñ�HCl��Һ��ϴ��ע���Һ����0���̶��� ��2��3cm�����ٰѵζ��̶ܹ��ã�����Һ������0���̶Ȼ�0���̶�����
E�����ζ����Ƿ�©ˮ
F����ȡ��ƿ�����ظ�����һ��
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶�
a���ζ���������ȷ˳���ǣ��������д��ECBADGF��
b����G���������ȷ���ζ��յ㣿���ν����һ����Һ�ɻ�ɫ���ɫ���Ұ�����ڲ���ɫ��
��2��������ԭ�ζ���ȡ������Һ����ƿ�У�������ϡ���ᣬ��Ũ��Ϊ0.1mol•L-1�ĸ��������Һ�ζ���������ӦΪ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+10CO2��+22MnSO4+8H2O�������¼��ʵ�����ݣ�
| �ζ����� | ����Һ��� ��mL�� | ��KMnO4��Һ�����mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
�ڸò�����Һ�����ʵ���Ũ��Ϊ0.2mol/L��
