��Ŀ����
10����ѧ������������������أ����ж��й�������ʵ�Ľ�����ȷ���ǣ�������| �������ʵ | ��Ҫԭ�� | |
| A | ȼú����������CaO�ɼ���SO2�ŷ��� | ȼ������Ԫ��ת��ΪCaSO3 |
| B | ������ϩ��Ĥ�������ڰ�װʳƷ | ����ɰ�ɫ��Ⱦ |
| C | �����������о��������ر������ | ������O3�������ӡ��������� |
| D | �����Ŵ�����ʵ�������ת��Ϊ����ɿڵ���� | ���ֵ���ˮ���������������Ҵ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��ȼ�պ���Ԫ���������ơ�������Ӧ����CaSO4��
B������ϩ�ж����������ڰ�װʳƷ��
C�������������״�������ת���ɳ�����
D������ˮ�����������ǣ���������һ��������ת�����Ҵ��Ͷ�����̼��
��� �⣺A����ú�в�����ʯ�ң����������뷴Ӧ���µ������£���������SO2��������ƣ����Լ���SO2���ŷţ���A����
B��������ϩ��Ĥ�������ڰ�װʳƷ��ԭ���Ǿ�����ϩ�ж���������Ϊ��ɰ�ɫ��Ⱦ����B����
C������������������O3�������ӡ��������٣����Ըо��������ر�����£���C��ȷ��
D�������Ŵ�����ʵ�������ת��Ϊ����ɿڵ���ƣ�������һ��������ת���������ǣ������ǽ�һ��ת�����Ҵ�����D����
��ѡC��
���� ���⿼�������ʵ���ɡ��ṹ�����ʵĹ�ϵ����Ŀ�Ѷ��еȣ��漰����ϩ��������Ⱦ������������ˮ�⡢���������ת����֪ʶ����ȷ����������ɺ�����Ϊ���ؼ����������������ѧ���ķ������������Ӧ��������

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
17�������£���0.1molNaHCO3��������ˮ���250mL��Һ�������¶Ȳ�������Һ�м����������ʣ��йؽ�����ȷ���ǣ�������
| ��������� | ���� | |
| A | ����Ba��OH��2 | ��Ӧ������c��CO32-����С |
| B | �ټ������Na2CO3 | ��Һ��c��CO32-����c��HCO3-�� |
| C | 100mLH2O | ��ˮ�������c��H+��•c��OH-������ |
| D | ������ | $\frac{c��C{O}_{3}^{2-}��}{c��HC{{O}_{3}}^{-}��}$���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
1��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ������������������Ҫ��������֣�H2S��HS-��S2-���ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ��ͼ��ʾ�����Եμӹ���H2S������ݳ���������˵������ȷ���ǣ�������
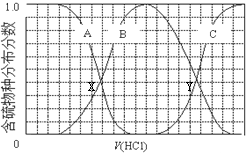

| A�� | ��������B��ʾHS- | |
| B�� | �ڵμ���������У���Һ��c��Na+���뺬�������Ũ�ȵĴ�С��ϵΪ��c��Na+��=3[c��H2S��+c��HS-��+c��S2-��] | |
| C�� | X��YΪ���ߵ�������㣬����֪��X�㴦��pH���Ϳ��Լ����H2S��Kaֵ | |
| D�� | NaHS�ʼ��ԣ�������Һ�м���CuSO4��Һ��ǡ����ȫ��Ӧ��������Һ��ǿ���ԣ���ԭ����Cu2++HS-�TCuS��+H+ |
18�������ס�����ԭ��������������ĵ�VA��Ԫ�أ����ǵĻ��������Ŷ��ص����ʺ���Ҫ��;��
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]3d104s24p3�������ס���ĵ�һ������˳��ΪN��P��As����Ԫ�ط��ţ���
��2����֪N2OΪֱ���νṹ���ṹʽΪN=N=O����N2O�Ǽ��ԣ�����ԡ��Ǽ��ԡ������ӣ��м�ĵ�ԭ�ӵ��ӻ��������Ϊsp�ӻ���
��3����֪�������ݣ�
NH3���ۡ��е������PH3��ԭ���ǰ�����֮�����γ������PH3�ķֽ��¶ȸ�����е㣬��ԭ����PH3�ֽ���Ҫ�ƻ�P-H����PH3������Ҫ�ƻ����Ӽ�������������ѧ���ȷ��Ӽ�������ǿ�ö࣮
��4�����ݼ۲���ӶԻ������ۣ��������еļ��ǣ������������������=����109��28�䣻PO43-���ӵĿռ乹��Ϊ�������壮
��5�������Ľṹ����ʯ���ƣ���ṹ��Ԫ��ͼ��ʾ��

�پ������㣬�þ���Ļ�ѧʽ��AlP��
�ڸþ������ǣ���ǡ���������λ����
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]3d104s24p3�������ס���ĵ�һ������˳��ΪN��P��As����Ԫ�ط��ţ���
��2����֪N2OΪֱ���νṹ���ṹʽΪN=N=O����N2O�Ǽ��ԣ�����ԡ��Ǽ��ԡ������ӣ��м�ĵ�ԭ�ӵ��ӻ��������Ϊsp�ӻ���
��3����֪�������ݣ�
| ���� | �۵�/K | �е�/K | �ֽ��¶�/K |
| NH3 | 195.3 | 239.7 | 1073 |
| PH3 | 139.2 | 185.4 | 713.2 |
��4�����ݼ۲���ӶԻ������ۣ��������еļ��ǣ������������������=����109��28�䣻PO43-���ӵĿռ乹��Ϊ�������壮
��5�������Ľṹ����ʯ���ƣ���ṹ��Ԫ��ͼ��ʾ��

�پ������㣬�þ���Ļ�ѧʽ��AlP��
�ڸþ������ǣ���ǡ���������λ����
5���������;�ܹ㷺��
��1����֪��
2NO2��g ���TN2O4��g����H=-56.9kJ•mol -1
H2O��g��=H2O��l����H=-44.0kJ•mol -1
CH4��g��+N2O4 ��g��=N2��g��+2H2O��l��+CO2 ��g����H=-898.1kJ•mol -1
�� CH4��g������ԭNO2 ��g������ N2��g����H2O��g�����Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g������H=-867kJ•mol-1��
��2��һ�������£�����2NO��g��+2CO��g��$\stackrel{����}{?}$N2��g��+2CO2��g����H��0���ڲ�ͬʱ��c��NO����c��CO�����±���ʾ��
�ٸ��¶��£��÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��{N}_{2}��{c}^{2}��C{O}_{2}��}{{c}^{2}��NO��{c}^{2}��CO��}$��
�ڼ���ǰ4���ڵ�����ƽ����Ӧ����Ϊ9.375��10-5mol/��L•s����
������ѡ���У�����˵��������Ӧ�Ѵ�ƽ�����AC
A��2v����NO��=v����N2 ��
B�������������ƽ������������ʱ����仯
C��������������ܶȲ���ʱ����仯
D��������CO���������ٷ����仯
E�� �������������ѹǿ����ʱ����仯
��3��CH4ȼ�ϵ��ԭ����ͼ��ʾ
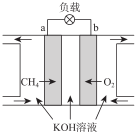
�ٸ�ȼ�ϵ�ص�����Ϊb���a����b�������õ�ظ����ĵ缫��ӦʽΪCH4+10OH--8e-=CO32-+7H2O��
�ڷŵ�һ��ʱ��������Һ��pH��С�����������С�����䡱����
��1����֪��
2NO2��g ���TN2O4��g����H=-56.9kJ•mol -1
H2O��g��=H2O��l����H=-44.0kJ•mol -1
CH4��g��+N2O4 ��g��=N2��g��+2H2O��l��+CO2 ��g����H=-898.1kJ•mol -1
�� CH4��g������ԭNO2 ��g������ N2��g����H2O��g�����Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g������H=-867kJ•mol-1��
��2��һ�������£�����2NO��g��+2CO��g��$\stackrel{����}{?}$N2��g��+2CO2��g����H��0���ڲ�ͬʱ��c��NO����c��CO�����±���ʾ��
| ʱ��/s | 0 | 2 | 4 | 6 | 8 | 10 |
| c��NO��/mol•L-1 | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��CO��/mol•L-1 | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
�ڼ���ǰ4���ڵ�����ƽ����Ӧ����Ϊ9.375��10-5mol/��L•s����
������ѡ���У�����˵��������Ӧ�Ѵ�ƽ�����AC
A��2v����NO��=v����N2 ��
B�������������ƽ������������ʱ����仯
C��������������ܶȲ���ʱ����仯
D��������CO���������ٷ����仯
E�� �������������ѹǿ����ʱ����仯
��3��CH4ȼ�ϵ��ԭ����ͼ��ʾ

�ٸ�ȼ�ϵ�ص�����Ϊb���a����b�������õ�ظ����ĵ缫��ӦʽΪCH4+10OH--8e-=CO32-+7H2O��
�ڷŵ�һ��ʱ��������Һ��pH��С�����������С�����䡱����
15��ͭ��ұ�����¿ɷ�Ϊ��
�ٸ��������������и�ѡ��
�ڱ��գ���Ҫ��ӦΪ2CuFeS2+4O2�TCu2S+3SO2+2FeO��¯������
���ƴ�ͭ����1200�淢������Ҫ��ӦΪ2Cu2S+3O2�T2Cu2O+2SO2��2Cu2O+Cu2S�T6Cu+SO2����
�ܵ�⾫��ͭ��
����˵������ȷ��������
�ٸ��������������и�ѡ��
�ڱ��գ���Ҫ��ӦΪ2CuFeS2+4O2�TCu2S+3SO2+2FeO��¯������
���ƴ�ͭ����1200�淢������Ҫ��ӦΪ2Cu2S+3O2�T2Cu2O+2SO2��2Cu2O+Cu2S�T6Cu+SO2����
�ܵ�⾫��ͭ��
����˵������ȷ��������
| A�� | �������չ��̵�β�����պ������������ | |
| B�� | ���������У���6molCuFeS2��ȡ6molCuʱ������15molO2 | |
| C�� | �ڷ�Ӧ2Cu2O+Cu2S=6Cu+SO2���У����������뻹ԭ��������ʵ�����1��6 | |
| D�� | �ڷ�Ӧ2Cu2O+Cu2S=6Cu+SO2���У�ֻ��Cu2O�������� |
19�������£�����������Һ��˵����ȷ���ǣ�������


| A�� | 25��ʱ��10-3mol/L�������pH=3��NH4Cl��Һ����pH=11�İ�ˮ�У�ˮ�ĵ���̶�Ϊ���ڣ��ۣ��� | |
| B�� | pH=1��NaHSO4��Һ��c��H+��=c��SO42-��+c��OH-�� | |
| C�� | pH=8.0��KHS��Һ�У�c��K+����c��HS-����c��OH-����c��S2-����c��H+�� | |
| D�� | ͼ��a����Һ�и�����Ũ�ȵĹ�ϵ�ǣ�c��OH-��=c��H+��+c��CH3COO-��+c��CH3COOH�� |
20����NA��ʾ�����ӵ�������ֵ��������������ȷ���ǣ�������
| A�� | 25��ʱ��pH=13�İ�ˮ��NaOH��Һ��1.0 L���е�OH-��Ŀ��Ϊ0.1NA | |
| B�� | ���³�ѹ�£���CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ����Ϊ2NA | |
| C�� | 22 gD218O�����У��������������������ֱ�Ϊ10NA��12NA | |
| D�� | ��״���£�22.4 L CCl4�к��е�C-Cl����ĿΪ4NA |

 ����ش��������⣺
����ش��������⣺ ��
�� �ṹ
�ṹ ��
��