��Ŀ����
4����֪��1273Kʱ������ӦFe��s��+H2O��g��?FeO��s��+H2��g�������ڹ̶��ݻ�Ϊ2.0L���ܱ������н��и÷�Ӧ���Խ���������⣺��1�������������䣬��С�������������Ӧ�������� �����������С�����䡱��
��2�������º�H2�İٷֺ������٣�������Ӧ�����ȷ�Ӧ��ѡ����ȡ��������ȡ�����
��3�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������b��
a����������ѹǿ���ֲ��� b��H2��Ũ�Ȳ��ٸı�
c������H2O�����ʵ���������H2�����ʵ���֮��Ϊ1��1
d��Fe��H2O��FeO��H2�����ʵ���֮��Ϊ1��1��1��1
��4������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g���TCH3OH��g������H=-90.8kJ•mol-1
��2CH3OH��g���TCH3OCH3��g��+H2O��g������H=-23.5kJ•mol-1
��CO��g��+H2O��g���TCO2��g��+H2��g������H=-41.3kJ•mol-1
�ܷ�Ӧ��3H2��g��+3CO��g���TCH3OCH3��g��+CO2 ��g���ġ�H=-246.4kJ/mol��
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��c������ĸ���ţ���
a�����¸�ѹ b��������� c������CO2��Ũ�� d������CO��Ũ�� e������������ѣ�
���� ��1����С�����������Ũ������Ӧ��������
��2�������º�H2�İٷֺ������٣�ƽ�����淴Ӧ�����ƶ���
��3���ﵽƽ��ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�Դ����������������䣬�Դ��жϣ�
��4�����ݸ�˹���ɣ��۲�Ŀ�귽��ʽ��֪���١�2+��+�ۣ����õ�3H2��g��+3CO��g��?CH3OCH3��g��+CO2 ��g�����ʡ�H=2��H1+��H2+��H3=-246.4kJ•mol-1��
��� �⣺��1����С�����������Ũ������Ӧ�������ʴ�Ϊ������
��2�������º�H2�İٷֺ������٣�ƽ�����淴Ӧ�����ƶ���������ȷ����ƶ���������Ӧ���ȣ��ʴ�Ϊ�����ȣ�
��3��a����Ӧǰ�������������䣬�����Ƿ�ﵽƽ��״̬����������ѹǿ���ֲ��䣬����˵���ﵽƽ��״̬����a����
b��H2��Ũ�Ȳ��ٸı䣬��˵���ﵽƽ��״̬����b��ȷ��
c������H2O�����ʵ���������H2�����ʵ���֮�ȣ�����ת�������ʵ���֮�ȵ��ڼ�����֮�ȣ�������˵���ﵽƽ��״̬����c����
d��Fe��H2O��FeO��H2�����ʵ���֮��ȡ����ת���ij̶ȣ�1��1��1��1����˵���ﵽƽ��״̬����d����
�ʴ�Ϊ��b��
��4����2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ•mol-1
��CO��g��+H2O ��g��?CO2��g��+H2��g������H=-41.3kJ•mol-1
���ݸ�˹���ɼ��㣬�١�2+��+�ۣ��õ��ܷ�Ӧ���Ȼ�ѧ����ʽ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H=-246.4kJ/mol��
a�������¶�����߷�Ӧ���ʣ���ƽ�����淴Ӧ�����ƶ���ת���ʼ�С����a����
b��������Ӱ��ƽ���ƶ��������������߷�Ӧ���ʣ����������CO��ת���ʣ���b����
c������CO2��Ũ�ȣ�ƽ��������Ӧ�ƶ���CO��ת��������c��ȷ��
d������CO��Ũ�ȣ���Ӧ��������ƽ��������Ӧ�ƶ�����CO��ת���ʼ�С����d����
e������������ѣ�ƽ��������Ӧ��Ӧ�ƶ�������Ӧ���ʼ�������e����
�ʴ�Ϊ��-246.4kJ/mol��c��
���� ���⿼���Ϊ�ۺϣ��漰��ѧ��Ӧ���ʵ�Ӱ�������Լ�ƽ��״̬���жϣ��ѶȲ���ע�������ػ���֪ʶ�Ļ��ۣ�

 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�| A�� | ���Ͻ� | B�� | ���Ͻ� | C�� | ͭ�Ͻ� | D�� | �ѺϽ� |
| A�� | �����ڲ����������--��ѧ��Ӧ����ЧӦ | |
| B�� | ���Ӽ��������Ĵ�С--�����ȶ��Եĸߵ� | |
| C�� | ԭ�Ӻ�������Ų�--Ԫ�������ڱ��е�λ�� | |
| D�� | ��Ч��ײ���ʵĸߵ�--��ѧ��Ӧ���ʵĴ�С |
 �����еĸ����������������ͼ�����߱�ʾ���ǣ�������
�����еĸ����������������ͼ�����߱�ʾ���ǣ������� | �� Ӧ | ������ | �� | �� | |
| A | ��ͬ�����İ�����ͬһ������ 2NH3�TN2+3H2 ��H��0 | ������ת���� | 500�� | 400�� |
| B | �������ء��Ʒֱ�������ˮ��Ӧ | H2���� | �� | �� |
| C | ���¶Ⱥ�ѹǿ����ͬ�ļ����������зֱ�Ͷ�������1��3��N2��H2��N2+3H2�T2NH3�� | ���������ʵ��� | ��Ӧ�����б��ֺ��º��� | �� Ӧ�����б��ֺ��º�ѹ |
| D | 2molSO2��1molO2������ͬ�¶��� 2SO2+O2�T2SO3 | SO3 ��Ũ�� | 2������ѹ | 10������ѹ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ����B��ת�������� | B�� | ƽ��������Ӧ�����ƶ� | ||
| C�� | ����A������������� | D�� | a+b��m+n |
 ������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е����λ����ͼ��ʾ������Dԭ�ӵ�����������M�������������������˵������ȷ���ǣ�������
������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е����λ����ͼ��ʾ������Dԭ�ӵ�����������M�������������������˵������ȷ���ǣ�������| A�� | A��5�����ۣ���B���γ�6�ֻ����� | |
| B�� | ��ҵ�ϳ�ͨ���������̬C2B3�ķ��������C�ĵ��� | |
| C�� | �����ӵİ뾶�ɴ�СΪ��E��A��B��C | |
| D�� | D��E��Ԫ���γɵĻ�����ÿ��ԭ������㶼�ﵽ��8e-�ȶ��ṹ |


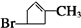
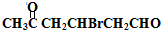 +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O
+Cu2O��+2H2O ��
��
 ����Ԫ�صĵ��ʼ��仯������ũҩ�����ʵȷ�������ҪӦ�ã����ǿ����������ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǣ�
����Ԫ�صĵ��ʼ��仯������ũҩ�����ʵȷ�������ҪӦ�ã����ǿ����������ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǣ�