��Ŀ����
4��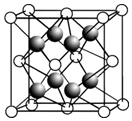 ��1������VSEPRģ���жϣ�������������ԭ�Ӷ���ͬһƽ���ϵ�һ����B��
��1������VSEPRģ���жϣ�������������ԭ�Ӷ���ͬһƽ���ϵ�һ����B��A��SO32-��NO2-B��NO3-��SO3
C��H3O+��ClO3-D��PO43-��SO32-
��2��CaF2�ľ�����ͼ��ʾ��
�������й�CaF2�ı�����ȷ����bd��
a��Ca2+��F-������ھ�����������
b��F-�����Ӱ뾶С��Cl-����CaF2���۵����CaCl2
c�����������ӱ�Ϊ2��1�����ӻ��������CaF2���幹����ͬ
d��CaF2�еĻ�ѧ��Ϊ���Ӽ������CaF2������״̬���ܵ���
��CaF2������ˮ���������ں�Al3+����Һ�У�ԭ����3CaF2+Al3+=[AlF6]3-+3Ca2+ �������ӷ���ʽ��ʾ����֪[AlF6]3-����Һ�п��ȶ����ڣ���
�۾�����F-����λ����4����һ��Ca2+�Ⱦ����������Ca2+��12����
��3��ͭ��п����Ԫ�صĵ�һ�����ܡ��ڶ������������ʾ
| ������/kJ•mol-1 | I1 | I2 |
| ͭ | 746 | 1958 |
| п | 906 | 1733 |
��ͭ�ĵ�һ�����ܣ�I1��С��п�ĵ�һ�����ܣ���ͭ�ĵڶ������ܣ�I2��ȴ����п�ĵڶ������ܣ�����Ҫԭ����Cuԭ��ʧȥһ�����Ӻ�������Ų�Ϊ[Ar]3d10����пΪ[Ar]3d104s1�����ݺ��ع���ͭ�ﵽ�˽��ȶ�״̬������Cu�ĵڶ���������Խϴ�
���� ��1������VSEPRģ���жϣ���������ԭ�Ӷ���ͬһƽ���ϣ�˵������Ϊƽ���νṹ��
��2����a���������Ӽ���ھ��������;����������
b�����Ӿ�����۵�������������ɡ����Ӱ뾶�йأ�
c������Ľṹ���ɱȡ��뾶���йأ�
d�����ӻ�����������ʱ�ܷ������룻
��F-��Al3+���γɺ��ѵ����������AlF63-��
�۾�����F-����λ����4����һ��Ca2+�Ⱦ����������Ca2+����=3��8��2��
��3����Cu��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д��̬Cuԭ�Ӻ�������Ų�ʽ��
�ݹ���е��Ӵ���ȫ����ȫ�ա�����ʱ���ȶ�����ʧȥ������Ҫ�������ϴ�
��� �⣺��1������VSEPRģ���жϣ���������ԭ�Ӷ���ͬһƽ���ϣ�˵������Ϊƽ���νṹ��
A��SO32-��Sԭ�Ӽ۲���ӶԸ���=3+$\frac{6+2-3��2}{2}$=4��Ϊ������ṹ��NO2-��Nԭ�Ӽ۲���ӶԸ���=2+$\frac{5+1-2��2}{2}$=3��Ϊ�����νṹ��������ƽ���νṹ��������A����
B��NO3-��Nԭ�Ӽ۲���ӶԸ���=3+$\frac{5+1-3��2}{2}$=3��Ϊƽ�������νṹ��
SO3��Sԭ�Ӽ۲���ӶԸ���=3+$\frac{6-3��2}{2}$=3��Ϊƽ�������νṹ������ԭ�ӹ�ƽ�棬��B��ȷ��
C��H3O+��Oԭ�Ӽ۲���ӶԸ���=3+$\frac{6-1-3��1}{2}$=4��Ϊ������ṹ��
ClO3-��Clԭ�Ӽ۲���ӶԸ���=3+$\frac{7+1-3��2}{2}$=4��Ϊ������ṹ������ԭ�Ӳ���ƽ�棬��C����
D��PO43-��Pԭ�Ӽ۲���ӶԸ���=4+$\frac{5+3-4��2}{2}$=4��Ϊ������ṹ��
SO32-��Sԭ�Ӽ۲���ӶԸ���=3+$\frac{6+2-3��2}{2}$=4��Ϊ������ṹ������ԭ�Ӳ���ƽ�棬��D����
��ѡB��
��2����a���������Ӽ���ھ��������;��������Ca2+��F-����ھ����������ã������ھ����������a����
b�����Ӿ�����۵�������������ɡ����Ӱ뾶�йأ����Ӱ뾶ԽС�����Ӿ�����۵�Խ�ߣ�����CaF2���۵����CaCl2����b��ȷ��
c������Ľṹ���ɱȡ��뾶���йأ��������ӱ�Ϊ2��1�����ʣ���CaF2����ĵ�ɱ���ͬ�����뾶�����ϴ����幹�Ͳ���ͬ����c����
d��CaF2�еĻ�ѧ��Ϊ���Ӽ������ӻ�����������ʱ�ܷ������룬���������ƶ������ӣ��ܵ��磬���CaF2������״̬���ܵ��磬��b��ȷ��
�ʴ�Ϊ��bd��
��CaF2������ˮ���������ں�Al3+����Һ�У���Ϊ����Һ��F-��Al3+���γɺ��ѵ����������AlF63-��ʹCaF2���ܽ�ƽ�����ƣ��䷴Ӧ�����ӷ���ʽΪ��3CaF2+Al3+=3Ca2++AlF63-��
�ʴ�Ϊ��3CaF2+Al3+=3Ca2++AlF63-��
�۾�����F-����λ����4����һ��Ca2+�Ⱦ����������Ca2+����=3��8��2=12��
�ʴ�Ϊ��4�� 12��
��3����Cu��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д��̬Cuԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ��[Ar]3d104s1��
��Cuԭ��ʧȥһ�����Ӻ�������Ų�Ϊ[Ar]3d10����пΪ[Ar]3d104s1�����ݺ��ع���ͭ�ﵽ�˽��ȶ�״̬������Cu�ĵڶ���������Խϴ�
�ʴ�Ϊ��Cuԭ��ʧȥһ�����Ӻ�������Ų�Ϊ[Ar]3d10����пΪ[Ar]3d104s1�����ݺ��ع���ͭ�ﵽ�˽��ȶ�״̬������Cu�ĵڶ���������Խϴ�
���� ���⿼����ۺϣ��漰�������㡢ԭ�Ӻ�������Ų����۲���ӶԻ������ۡ����Ӿ����֪ʶ�㣬���ؿ���ѧ���������жϡ�֪ʶ�ۺ�Ӧ�á��ռ����������ȣ�ע��۲���ӶԻ��������ں���ԭ�Ӻ�������Ų������ȶ��Թ�ϵ����Ŀ�ѶȲ���

����˵���д�����ǣ�������
| A�� | KClO3�ڷ�Ӧ�еõ����� | |
| B�� | ClO2���ȵĻ��ϼ�Ϊ+4�� | |
| C�� | �ڷ�Ӧ��H2C2O4�ǻ�ԭ�� | |
| D�� | 1 mol KClO3�μӷ�Ӧ��2mol����ת�� |
| A�� | ��״���£�a L�������͵����Ļ���ﺬ�еķ�����ԼΪ$\frac{a}{22.4}$��6.02��1023 | |
| B�� | ��1L 1 mol•L-1�� FeCl3��Һ�ƳɵĽ����н�������С��NA�� | |
| C�� | 2.3g�����Ʊ�Ϊ������ʧȥ0.1NA���� | |
| D�� | 2.24L CO2�к��е�ԭ����Ϊ3��0.1��6.02��1023 |
CrO${\;}_{4}^{2-}$$��_{��ת��}^{H+}$Cr2O${\;}_{7}^{2-}$$��_{�ڻ�ԭ}^{Fe_{2}+}$Cr3+$��_{�۳���}^{OH-}$Cr��OH��3��
��֪��
��1��������д���ƽ�⣺2Cr O42-����ɫ��+2H+?Cr2O72-����ɫ��+H2O
��2����������ɵ�Cr��OH��3 ����Һ�д��ڳ����ܽ�ƽ�⣺Cr��OH��3��s��?Cr3+��aq��+3OH һ��aq��
��3�������£�Cr��OH��3 ���ܶȻ�Ksp=10-32���ҵ���Һ������Ũ��С��10-5 mol•L-1 ʱ�����������Ӳ����ڣ������й�˵���У���ȷ���ǣ�������
| A�� | ������м��ᣬ����Һ��pH ������2����Һ�Ի�ɫ��CrO42-����Ũ������ | |
| B�� | ������е���Һ�������ɫ����2v��CrO${\;}_{4}^{2-}$��=v��Cr2O72-��ʱ��˵����Ӧ2CrO42-����ɫ��+2H+?Cr2O72-����ɫ��+H2O �ﵽƽ��״̬ | |
| C�� | ������У���Ҫ��ԭ1 mol Cr2O${\;}_{7}^{2-}$���ӣ���Ҫ6 mol��NH4��2Fe��SO4��2•6H2O | |
| D�� | ������У�������Һ��pH ������4 ʱ������Ϊ��ˮ�еĸ�Ԫ���ѻ������� |
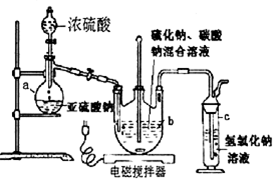
�ٿ�����Һ©����ʹ�����������£��ʵ����������У�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ������Ž�����������
����������������ʧ��������Һ��pH�ӽ�7ʱ��ֹͣͨ��SO2���壮
�۳������õ���Һ��ת�����������У�ˮԡ����Ũ����ֱ����Һ������־�Ĥ��
����ȴ�ᾧ�����ˡ�ϴ�ӣ�
�ݽ������������У���40��45�����Ҹ���50��60min��������
��ش��������⣺
��l������a��������������ƿ��
��2���������������pHֵС��7������ʻ��½����������ӷ���ʽ����ԭ��S2O32-+2H+=S��+H2O+SO2����
��3��������в��ܽ���Һ�������ɵ�ԭ�������ɻ�ʹ�����������ˮ���ֽ⣻��Ĥͨ������Һ������ֵ�ԭ������Ϊ��Һ�����¶Ƚϵͣ�
��4���������ϴ����������ƾ��������Լ��Ľṹʽ��
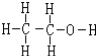 ��
����5��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ5��0�˵IJ�Ʒ���Ƴ�250mL�����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���25mL 0.0lmol•L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+S4O62-������ɫ��ȥH����Ӳ���ɫʱ����ζ��յ㣮ʵ���������±���
| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
A���ζ���ĩ��Na2S2O3��Һ��ϴ B���ζ��յ�ʱ���Ӷ���
C����ƿ������ˮ��ϴ D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢�����ݣ�
 298Kʱ����20.0mL0.20mol•L-1��ˮ�е���0.20mol•L-1�����ᣬ��Һ��pH����������������ϵ��ͼ��ʾ��
298Kʱ����20.0mL0.20mol•L-1��ˮ�е���0.20mol•L-1�����ᣬ��Һ��pH����������������ϵ��ͼ��ʾ��