��Ŀ����
12���ӱ������еõ�һ����A��C10H16�����Ц�����������A��ط�Ӧ��ͼ��
��֪��

��1��H�ķ���ʽΪC10H20��
��2��B���������ŵ�����Ϊ�ʻ����Ȼ���
��3��������-COOCH3���ŵ�C��ͬ���칹�干��4�֣������������칹�������к˴Ź������׳���2�����շ���칹��Ľṹ��ʽΪ
 ��
����4��B��D��D��E�ķ�Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ȡ����Ӧ��
��5��GΪ����Ԫ���Ļ����д����ṹ��ʽ��
 ��
����6��д��E��F�Ļ�ѧ��Ӧ����ʽ��
 ��
����7��A�Ľṹ��ʽΪ
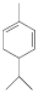 ��A������ʵ�����Br2���мӳɷ�Ӧ�IJ��ﹲ��3�֣������������칹����
��A������ʵ�����Br2���мӳɷ�Ӧ�IJ��ﹲ��3�֣������������칹����
���� ��A��H��ת�����⣬�л�����ӵ�̼���Ǽ�û�б仯����֪A�����к���һ����Ԫ�������A�ķ���ʽC10H16��֪��Ӧ�������������Ͷȣ��ٸ���������Ϣ�Լ�C�Ľṹ��ʽ��B�ķ���ʽ���Ƴ�A�Ľṹ��ʽΪ ����B�Ľṹ��ʽΪ
����B�Ľṹ��ʽΪ ��B��D���⣬���ڼӳɷ�Ӧ����DΪ����
��B��D���⣬���ڼӳɷ�Ӧ����DΪ���� ����E��F���������������ƵĴ���Һ���ȡ���ӦΪ±��������ȥ�����ƿ�֪D��E����ȡ����Ӧ����ԭ��ȡ������������е��ǻ�����E��F�Ľṹ��ʽ�ֱ�Ϊ
����E��F���������������ƵĴ���Һ���ȡ���ӦΪ±��������ȥ�����ƿ�֪D��E����ȡ����Ӧ����ԭ��ȡ������������е��ǻ�����E��F�Ľṹ��ʽ�ֱ�Ϊ ��
�� ��GΪ����D������������ˮ�γɣ��ṹ��ʽΪ
��GΪ����D������������ˮ�γɣ��ṹ��ʽΪ ���ݴ˷������
���ݴ˷������
��� �⣺��A��H��ת�����⣬�л�����ӵ�̼���Ǽ�û�б仯����֪A�����к���һ����Ԫ�������A�ķ���ʽC10H16��֪��Ӧ�������������Ͷȣ��ٸ���������Ϣ�Լ�C�Ľṹ��ʽ��B�ķ���ʽ���Ƴ�A�Ľṹ��ʽΪ ����B�Ľṹ��ʽΪ
����B�Ľṹ��ʽΪ ��B��D���⣬���ڼӳɷ�Ӧ����DΪ����
��B��D���⣬���ڼӳɷ�Ӧ����DΪ���� ����E��F���������������ƵĴ���Һ���ȡ���ӦΪ±��������ȥ�����ƿ�֪D��E����ȡ����Ӧ����ԭ��ȡ������������е��ǻ�����E��F�Ľṹ��ʽ�ֱ�Ϊ
����E��F���������������ƵĴ���Һ���ȡ���ӦΪ±��������ȥ�����ƿ�֪D��E����ȡ����Ӧ����ԭ��ȡ������������е��ǻ�����E��F�Ľṹ��ʽ�ֱ�Ϊ ��
�� ��GΪ����D������������ˮ�γɣ��ṹ��ʽΪ
��GΪ����D������������ˮ�γɣ��ṹ��ʽΪ ��
��
��1������H�Ľṹ��ʽ�ɵ÷���ʽΪC10H20���ʴ�Ϊ��C10H20��
��2��B�Ľṹ��ʽΪ ������B���������ŵ�����Ϊ�ʻ����Ȼ����ʴ�Ϊ���ʻ����Ȼ���
������B���������ŵ�����Ϊ�ʻ����Ȼ����ʴ�Ϊ���ʻ����Ȼ���
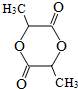
��3������ �����ڶ˵㣬�ʿ�д��������������ͬ���칹���У�������-COOCH3���ŵ�C��ͬ���칹�干��4�֣�
�����ڶ˵㣬�ʿ�д��������������ͬ���칹���У�������-COOCH3���ŵ�C��ͬ���칹�干��4�֣� ��CH3OOCCH2CH2CH2COOCH3��CH3OOCCH2CH��CH3��CH2COOCH3��CH3CH2CH��COOCH3��2���˴Ź������׳���2�����շ壬��ԭ��������λ�ã��ṹ��ʽΪ
��CH3OOCCH2CH2CH2COOCH3��CH3OOCCH2CH��CH3��CH2COOCH3��CH3CH2CH��COOCH3��2���˴Ź������׳���2�����շ壬��ԭ��������λ�ã��ṹ��ʽΪ ���ʴ�Ϊ��4��
���ʴ�Ϊ��4�� ��
��
��4��B��DΪ�ʻ������������ļӳɷ�Ӧ��D��EΪ��ԭ�ӱ���ԭ��ȡ������Ӧ����Ϊȡ����Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
��5��D�������ǻ����Ȼ�����������Ӧ����G��G�Ľṹ��ʽΪ ���ʴ�Ϊ
���ʴ�Ϊ ��
��
��6��E���������ƴ���Һ������ȥ��Ӧ���кͷ�Ӧ������E��F�Ļ�ѧ��Ӧ����ʽ ��
��
�ʴ�Ϊ ��
��
��7������B��C�Ľṹ��ʽ��A�ķ���ʽC10H16���Ƴ�A�Ľṹ��ʽΪ ��A������̼̼˫��������ʵ������嵥�ʿɷֱ���мӳɷ�Ӧ��Ҳ�ɷ���1��4�ӳɣ����Բ��ﹲ��3�֣��ʴ�Ϊ��
��A������̼̼˫��������ʵ������嵥�ʿɷֱ���мӳɷ�Ӧ��Ҳ�ɷ���1��4�ӳɣ����Բ��ﹲ��3�֣��ʴ�Ϊ�� ��3��
��3��
���� ���⿼���л��ϳɵķ������ƶϣ���Ŀ�ѶȲ���ע����ճ����л���Ĺ����ŵ����ʣ���ס��Ӧ�����������л��ﷴӦ�Ļ�ѧ����ʽҪ��д���ѵ���ͬ���칹�������жϣ�

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�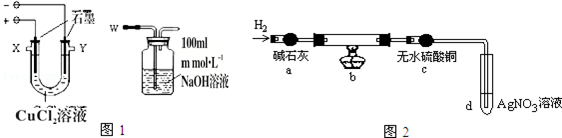
��1��������W��Ӧ�������X���ӣ��X����Y������
��2��ʵ���������̼���ϳ��˸����к�ɫ���ʣ���������������ɫ���ʣ�����������ʾ��
| �������Ƽ���ѧʽ | �Ȼ���ͭCuCl | ��ʽ�Ȼ�ͭCu2��OH��3Cl |
| ���� | ��ɫ���塢����ˮ | ��ɫ���塢����ˮ |
�ں�ɫ���ʿ�����Cu������Cu2O�����߶�����
ʵ���Ϊ̽������̼���ϸ��ŵĺ�ɫ����ɫ���ʣ����������ʵ�飺ȡ������̼����ϴ�ӡ������������ΪW1g�����������ͼ2��ʾװ��b�У�����ʵ�飮
ʵ���У�̼���ϵİ�ɫ������ȫ��Ϊ��ɫ����ˮ����ͭ����ɫ��d�г��ְ�ɫ������ʵ�����ʱ������ͨH2ֱ��̼����ȴ����������ΪW2g��
��3����ˮ����ͭ�������Ǽ����ɫ����������Cu2O��
��4��װ��b�з�����Ӧ�Ļ�ѧ����ʽ��2CuCl+H2=2Cu+2HCl��
��5�����CuCl2��Һʱ�������ϲ�����ɫ���ʵ�ԭ��Ϊ���õ缫��Ӧʽ���ͣ�Cu2++e-+Cl-=CuCl���������ϲ�����ɫ���ʵ����ʵ�����$\frac{W{\;}_{1}-W{\;}_{2}}{35.5}$mol��
ʵ���ⶨƯ��Һ��NaClO��Ũ�ȣ�ȷ��ȡ20.00mLϴ��ƿ�ڻ����Һ������������H2O2��Һ��ҡ�ȣ��μ�2��3�η�̪��Һ���� n mol•L-1����ζ����յ㣬��������V mL��
��6���û�ѧ����ʽ��ʾ����H2O2��Һ������NaClO+H2O2=O2��+NaCl+H2O��
��7��������ϴ��ƿ�ڵĸ���Ӧ������仯��Ư��Һ��NaClO��Ũ��Ϊ$\frac{20m-nV}{40}$mol•L-1��
| A�� | ��������þ | B�� | ����̼��� | ||
| C�� | ��ⱥ���Ȼ�����Һ | D�� | ��������Ȼ�þ |
| A�� | ŨHCl��ŨH2SO4��ŨHNO3�����������ԣ������������� | |
| B�� | ���ݷ�ɢϵ�Ƿ��ж����ЧӦ����ɢϵ��Ϊ��Һ���������Һ | |
| C�� | NaOH��HNO3��NaNO3��ˮ��Һ�о��ܵ�������ӣ��������ӻ����� | |
| D�� | Na2O2��HCl��BaSO4������״̬������ˮʱ���ܵ��磬��������� |
| A�� | �ϳ���ά���ά�������������ǽ������� | |
| B�� | ���ع��͡���ֹʳ�ã���������T��������������ȡ������������� | |
| C�� | �����ƹ�Ӧ�á������������������ɼ������������������Կ�������Ⱦ | |
| D�� | 2015��10���ҹ���ѧ�������ϻ�ŵ����ҽѧ������ѧ���������֡����Ƶ�ҩ������أ� ��������ű������Чҩ������صķ���ʽΪC15H22O5 ��������ű������Чҩ������صķ���ʽΪC15H22O5 |
| A�� | pH=1����ɫ��Һ�У�SO42����Cu2+��Na+��Cl�� | |
| B�� | ������Һ�У�Fe3+��Al3+��NO3����Cl�� | |
| C�� | ��ʹʯ����ֽ����ɫ����Һ�У�Na+��K+��S2����CO32�� | |
| D�� | ˮ�������c��H+��=1��10��12mol•L��1����Һ�У�K+��Na+��Cl����HCO3�� |
 ��1��ʵ���ҳ���װ��E�Ʊ�Cl2��д���÷�Ӧ�����ӷ���ʽ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
��1��ʵ���ҳ���װ��E�Ʊ�Cl2��д���÷�Ӧ�����ӷ���ʽ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O�� ��
��