��Ŀ����
7����б������������������ͬ����������֪����S����б��s��+O2��g���TSO2��g����H1=-297.16kJ•mol-1
��S��������s��+O2��g���TSO2��g����H2=-296.83kJ•mol-1
��S�����s���TS��������s����H3
����˵����ȷ���ǣ�������
| A�� | ��H3=+0.33 kJ•mol-1 | |
| B�� | ��б��ת��Ϊ������ķ�Ӧ�����ȷ�Ӧ | |
| C�� | ��H3��0��������ȵ�б���ȶ� | |
| D�� | ��H3��0����б����������ȶ� |
���� A�����ݸ�˹���ɿ�֪����Ӧ��-��=�ۣ����ԡ�H3=��H1-��H2��
B�����ݷ�Ӧ���ʱ�����ȷ����Ӧ���ȡ����������
C�����������ߵ����ʲ��ȶ��������͵������ȶ����ش�
D�����������ߵ����ʲ��ȶ��������͵������ȶ����ش�
��� �⣺A���ɸ�˹���ɿ�֪����Ӧ��-��=�ۣ����ԡ�H3=��-297.16kJ•mol-1��-��-296.83kJ•mol-1��=-0.33kJ/mol����A����
B��S����б��s���TS��������s����H3=-0.33kJ/mol����Ӧ���ȣ���B����
C������S����б��s���TS��������s����H3=-0.33kJ/mol����б���������������������ߣ�������ȵ�б���ȶ�����C��ȷ��
D������S����б��s���TS��������s����H3=-0.33kJ/mol����б���������������������ߣ�������ȵ�б���ȶ�����D����
��ѡ��C��
���� ���⿼�黯ѧ�������ܣ���ȷ��˹���ɼ��㷴Ӧ�ȼ����ʵ��������ȶ��ԵĹ�ϵ���ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14�����Ӿ���֮��ľ��������Ӽ��Ĵ�С�����а����Ӽ��ɴ�С���е��ǣ�������
| A�� | NaF��NaCl��NaBr��NaI | B�� | Na20��Na2S��NaCl��NaI | ||
| C�� | NaCl��CaCl2��MgCl2��AlCl3 | D�� | MgCO3��CaCO3��SrCO3��BaCO3 |
18����ѧ��Ϊ�˷��㣬��������ƽ�ⳣ���ĸ�������ΪpKa����pKa=-lgKa���������̼���pKa�������£�
��1��0.01mol/L NaHSO3��Һ��pH=bl��0.01mol/L NaHCO3��Һ��pH=b2��b1��b2��ѡ�����������������=������
��2����10mL 0.01mol/L��H2SO3��Һ�У��μ�0.0lmol/L KOH��ҺV��mL����
�ٵ�V=10mLʱ����Һ�д��ڣ�c��K+����c��HSO3-����c��SO32-����c��H2SO3������c��H+����c��OH-����ѡ�����������������=������
�ڵ�V=a mLʱ����Һ������Ũ�������¹�ϵ��c��K+��=2c��SO32-��+c��HSO3-��������Һ�����ԣ�ѡ��ᡱ�����С������������V=b mLʱ����Һ������Ũ�������¹�ϵ��c��K+��=c��SO32-��+c��HSO3--��+c��H2SO3��������Һ�����ԣ�ѡ��ᡱ�����С������������a��b��ѡ�����������������=������
| ���� | H2SO3 | HSO3- | H2CO3 | HCO3- |
| PKa | 1.9 | 7.2 | 6.4 | 10.3 |
��2����10mL 0.01mol/L��H2SO3��Һ�У��μ�0.0lmol/L KOH��ҺV��mL����
�ٵ�V=10mLʱ����Һ�д��ڣ�c��K+����c��HSO3-����c��SO32-����c��H2SO3������c��H+����c��OH-����ѡ�����������������=������
�ڵ�V=a mLʱ����Һ������Ũ�������¹�ϵ��c��K+��=2c��SO32-��+c��HSO3-��������Һ�����ԣ�ѡ��ᡱ�����С������������V=b mLʱ����Һ������Ũ�������¹�ϵ��c��K+��=c��SO32-��+c��HSO3--��+c��H2SO3��������Һ�����ԣ�ѡ��ᡱ�����С������������a��b��ѡ�����������������=������
15����pH=2������Һ��pH=12�ļ���Һ�������Ϻ���Һ�ʼ��ԣ���ԭ����ܣ�������
| A�� | ��Ӧ����ˮ��ʹ��Һ�ʼ��� | B�� | ������Һ��ǿ����Һ��Ӧ | ||
| C�� | ǿ����Һ��������Һ��Ӧ | D�� | һԪǿ����Һ��һԪǿ����Һ��Ӧ |
2�����еĸ���������������Һ���ܴ���������ǣ�������
| A�� | Ca2+��K+��Cl-��NO3- | B�� | Na+��Ca2+��SO32-��ClO- | ||
| C�� | Al3+��K+��OH-��NO3- | D�� | Na+��Ca2+��SiO32-��Cl- |
12��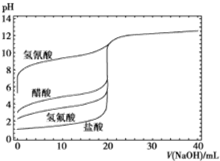 ��ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ�������
��ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ�������
 ��ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ�������
��ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ�������| A�� | ���ԣ�HF��CH3COOH��HCN | |
| B�� | ��NaOH��Һ�ζ�����ʱ��Ӧ�÷�̪��ָʾ������ʹ�ü��� | |
| C�� | ������10mLNaOH��Һʱ��c��CN-����c��CH3COO-�� | |
| D�� | ��NaOH��Һ�ĵ��룬CH3COOH��Һ��ˮ�ĵ���̶��ȱ����С |
19��������̼�IJ�����������������Դ�����һ����Ҫ�о�����
��1����̫���������£���CO2Ϊԭ����ȡ̼��C����������ͼ1��ʾ��

���ȷֽ�ϵͳÿ�ֽ�0.5mol Fe3O4ת�Ƶ��ӵ����ʵ���Ϊ1mol��
�ڸ�ϵͳ���ܷ�Ӧ�Ļ�ѧ����ʽ��CO2�TC+O2��
��2��������̼������ɺϳɵ�̼ϩ��
2CO2��g��+6H2��g��?C2H4��g��+4H2O��g����H1=-a kJ•mol-1���й������仯��ͼ2��ʾ��
�ټ������ʾ��е��������ڱ�״���£��涨���ʵ�����Ϊ0�������������������ʱ�ų������յ�������Ϊ����е������������ʾ��
��a=128
�ڼ��ֻ�ѧ���ļ������£�
��b=436
��3���ݱ����Զ�����̼Ϊԭ�ϲ�������ĵ缫���ǿ���ԵĶ�����̼ˮ��Һ�ɵõ�����ȼ�ϣ���ԭ����ͼ����ʾ��

�ٸù���������ת����ʽ��Ҫ��̫����ת��Ϊ���ܡ�����ת��Ϊ��ѧ�ܣ�д������������ʽ���ɣ���
������b�������ɱ�ϩ��CH3CH=CH2���ĵ缫��ӦʽΪ3CO2+18H++18e-=CH3CH=CH2+6H2O��
����ϩ��������Ӧͨ����ͼ��װ�ÿɽ���ѧ��ת��Ϊ���ܣ�b�缫��ӦʽΪO2+2H2O+4e-=4OH-��
��1����̫���������£���CO2Ϊԭ����ȡ̼��C����������ͼ1��ʾ��

���ȷֽ�ϵͳÿ�ֽ�0.5mol Fe3O4ת�Ƶ��ӵ����ʵ���Ϊ1mol��
�ڸ�ϵͳ���ܷ�Ӧ�Ļ�ѧ����ʽ��CO2�TC+O2��
��2��������̼������ɺϳɵ�̼ϩ��
2CO2��g��+6H2��g��?C2H4��g��+4H2O��g����H1=-a kJ•mol-1���й������仯��ͼ2��ʾ��
�ټ������ʾ��е��������ڱ�״���£��涨���ʵ�����Ϊ0�������������������ʱ�ų������յ�������Ϊ����е������������ʾ��
| ���� | CO2��g�� | C2H4��g�� | H2O��g�� |
| ����/kJ•mol-1 | -394 | 52 | -242 |
�ڼ��ֻ�ѧ���ļ������£�
| ��ѧ�� | C=O | H-H | C=C | O-H |
| ����/kJ•mol-1 | 803 | b | 615 | 463 |
��3���ݱ����Զ�����̼Ϊԭ�ϲ�������ĵ缫���ǿ���ԵĶ�����̼ˮ��Һ�ɵõ�����ȼ�ϣ���ԭ����ͼ����ʾ��

�ٸù���������ת����ʽ��Ҫ��̫����ת��Ϊ���ܡ�����ת��Ϊ��ѧ�ܣ�д������������ʽ���ɣ���
������b�������ɱ�ϩ��CH3CH=CH2���ĵ缫��ӦʽΪ3CO2+18H++18e-=CH3CH=CH2+6H2O��
����ϩ��������Ӧͨ����ͼ��װ�ÿɽ���ѧ��ת��Ϊ���ܣ�b�缫��ӦʽΪO2+2H2O+4e-=4OH-��
16�����б�ʾʽ������ǣ�������
| A�� | Al3+�Ľṹʾ��ͼ�� | B�� | �Ȼ��Ƶĵ���ʽ��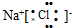 | ||
| C�� | �����ӵĵ���ʽ��Al3+ | D�� | ��ԭ�ӵĽṹʾ��ͼ�� |