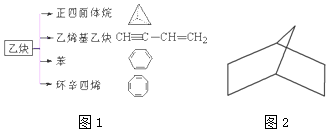
��Ŀ����
18����NA��ʾ����٤��������ֵ����ش��������⣺��1��12.4gNa2X����0.4molNa+����Na2X��Ħ������Ϊ62g/mol�������ԭ������Ϊ62��X�����ԭ������Ϊ16��Na2X�Ļ�ѧʽΪNa2O
��2��ͬ��ͬѹ�£�һ���������̬�⻯��HmX�������ǵ����NH3��2������X�����ԭ������Ϊ34-m ���ú�m�Ĵ���ʽ��ʾ����
��3����ͬ�����£�ͬ������X��Y�������壬��Է��������ֱ�ΪA��B����
��X��Y�������ΪB��A�����Ӹ�����ΪB��A���ܶȱ�ΪA��B��
����ͬ�����µ�X��Y�������������ͬ����X��Y��������ΪA��B�����ʵ�����Ϊ1��1��
���� ��1��12.4��Na2X�к���0.4molNa+������Na2X����ɼ����Na2X�����ʵ�����Ħ������������Ħ����������Է������Ĺ�ϵ�����X�����ԭ������������Na2X���ԭ��������ȷ������ʽ��
��2��ͬ��ͬѹ�£��ܶ�֮�ȵ�����Է�������֮�ȣ��ݴ˼���H2X����Է�����������������X�����ԭ��������
��3����ͬ��ͬѹ�£��������֮�ȵ������ʵ���֮�ȣ����n=$\frac{m}{M}$�ж����֮�ȣ�ͬ��ͬѹ�£������ܶ�֮�ȵ�������Է�������֮�ȣ�
��ͬ��ͬѹ�£��������֮�ȵ������ʵ���֮�ȣ����m=nM�ж϶�������֮�ȣ�
��� �⣺��1��12.4��Na2X�к���0.4molNa+��Na2X�����ʵ���Ϊ��n��Na2X��=$\frac{1}{2}$n��Na+��=0.4mol��$\frac{1}{2}$=0.2mol��
Na2X��Ħ������Ϊ��M��Na2X��=$\frac{12.4g}{0.2mol}$=62g/mol��
Ħ����������ֵ�ϵ�������Է�����������Na2X�����ԭ����Ϊ62��
��ԭ�ӵ����ԭ��������23������X�����ԭ��������62-23��2=16��
XΪ��ԭ�ӣ������ʵĻ�ѧʽΪNa2O��
�ʴ�Ϊ��62g/mol��62��16��Na2O��
��2����ͬ״���£�һ���������̬�⻯��HmX�������ǵ����NH3��2����������ܶ�֮��Ϊ2��1��ͬ��ͬѹ�£��ܶ�֮�ȵ�����Է�������֮�ȣ�HmX����Է�������Ϊ17��2=34����X�����ԭ������Ϊ34-m��
�ʴ�Ϊ��34-m��
��3���ٸ���n=$\frac{m}{M}$��֪��ͬ������X��Y�������ʵ���֮����Ħ�������ɷ��ȣ���ͬ������X��Y�������ʵ���֮��=B��A��ͬ��ͬѹ�£��������֮�ȵ������ʵ���֮��=B��A��
ͬ��ͬѹ�£������ܶ�֮�ȵ�������Է�������֮��=A��B��
�ʴ�Ϊ��B��A��B��A��A��B��
��ͬ��ͬѹ�£����ʵ���֮�ȵ������֮�ȣ�����ͬ�����X��Y�����ʵ���֮��=1��1�����m=nM��֪����������֮��=Ħ������֮��=A��B��
�ʴ�Ϊ��A��B��1��1��
���� ���⿼�������ʵ����ļ��㣬��Ŀ�ѶȲ�����ȷ���ʵ�����Ħ������������٤������֮��Ĺ�ϵΪ���ؼ����������������ѧ���ķ�����������ѧ����������

| A�� | 56g��������������ˮ������ַ�Ӧת�Ƶ�����ĿΪ3NA | |
| B�� | 100 g CaCO3��KHCO3��Ϲ�����CO32-����ĿΪNA | |
| C�� | ʵ������H2O2�Ʊ�1mol O2ת�Ƶĵ�����Ϊ2NA | |
| D�� | ��״���£�2.24L CCl4���е�ԭ����Ϊ0.5NA |
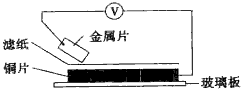 ���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������
���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������ | ���� | ������������ | ��ѹ |
| �� | �ס�Cu | +0.78 |
| �� | Cu���� | +0.15 |
| �� | ����Cu | +1.35 |
| �� | ����Cu | +0.30 |
| A�� | �����ֽ������ҵĻ�ԭ����ǿ | |
| B�� | �������ܴ�����ͭ��Һ���û���ͭ | |
| C�� | �ס������γ�ԭ���ʱ����Ϊ���� | |
| D�� | �ס����γɺϽ��ڿ����У����ȱ���ʴ |
| A�� | һ�������£�2molSO2 �� 1molO2 ������ܱ������г�ַ�Ӧ�������еķ���������2NA | |
| B�� | 256g S8 �����к� S-S ��Ϊ 7NA �� | |
| C�� | �� 1molCH3COONa ������ CH3COOH �γɵ�������Һ�У�CH3COO-��ĿΪ NA �� | |
| D�� | 1 mol Na �� O2 ��ȫ��Ӧ������ NaO2 �� Na2O2�Ļ���ת�Ƶ�������Ϊ NA �� |
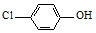 ���ķ�ˮ�������������س�ȥ����ԭ����ͼ1��ʾ��
���ķ�ˮ�������������س�ȥ����ԭ����ͼ1��ʾ��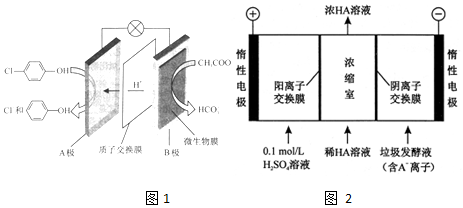
 -OH+2e-+H+�T
-OH+2e-+H+�T -OH+Cl-
-OH+Cl-