��Ŀ����
8����֪����SO2ͨ��FeCl3��Һ�У���Һ��ɫ���Ϊdz��ɫ����ԭ���ɱ�ʾΪ����Fe3++��SO2+��H2O--��Fe2++��SO42-+��H+
��1��������������������ƽ���ϵ�������õ����ŷ��������ת�Ƶķ������Ŀ��
��2�����μӷ�Ӧ��SO2���Ϊ1.12L����״���£�����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ0.1mol��
��3����֪��Fe2+��Һ�еμ���������ʱ����Һ��dz��ɫ��Ϊ��ɫ����Fe3+��SO42-��HNO3����������ǿ������˳��ΪHNO3��Fe3+��SO42-����
���� ��1�������ϼ���������ƽ����ʽ��
��2�������������������ʵ���������SO2$\stackrel{2{e}^{-}}{��}$SO42-����ת�Ƶ�������
��3������Һ��dz��ɫ��Ϊ��ɫ��˵���������ӱ�����Ϊ�����ӣ������������������Դ�����������������Կ��жϣ�����������ǿ�����������ӣ�Fe3+��SO42-������ǿ���ɴӣ�1���õ���
��� �⣺��1��Fe3+��Fe2+�����ϼ۴�+3��+2������1�ۣ�SO2��SO42-�����ϼ۴�+4��+6������2�ۣ����ϼ����ߺͽ���������ȣ�
����Ԫ��ǰ��ϵ��Ϊ2����Ԫ��ǰ��ϵ��Ϊ1�����ٸ�����ԭ���غ��֪H2Oǰ��ϵ��Ϊ2��H+ǰϵ��Ϊ4������ת�Ƶķ������ĿΪ�� ���ʴ�Ϊ��2��1��2��2��1��4��
���ʴ�Ϊ��2��1��2��2��1��4�� ��
��
��2��SO2�����ʵ���Ϊ$\frac{1.12L}{22.4L/mol}$=0.05mol������SO2$\stackrel{2{e}^{-}}{��}$SO42-����ת�Ƶ�������0.05mol��2=0.1mol��
�ʴ�Ϊ��0.1��
��3��dz��ɫΪ����������ɫ����ɫΪ������������ɫ����Һ��dz��ɫ��Ϊ��ɫ��˵���������ӱ�����Ϊ�����ӣ�������������ǿ�����������ӣ����ݣ�1����֪������������������ǿ��SO42-���ʴ�Ϊ��HNO3��Fe3+��SO42-��
���� ���⿼����������ԭ��Ӧ����ƽ��������ǿ���Ƚϣ��е��Ѷȣ�ע����ƽһ���û��ϼ���������ƽ���Ԫ�أ��ٸ���ԭ���غ���ƽ����Ԫ�أ����ݷ�Ӧ����ʽ�ж������ԣ��������������Դ�����������������ԣ�

| ��ѧ�� | H-H | C-H | C��C |
| ����/kJ•mol-1 | a | b | c |
| A�� | ��b-c-a��kJ•mol-1 | B�� | ��c+3a-4b��kJ•mol-1 | C�� | ��6b-c-3a��kJ•mol-1 | D�� | ��c+3a-6b��kJ•mol-1 |
| A�� | ú��������Һ�����������仯����ú�м�������CaSO4���ɴ����ٲ�����SO2���� | |
| B�� | BaSO4��ҽѧ���������ͣ�Ba2+�������� | |
| C�� | PM 2.5��������̼�����ڿ��������ձ������� | |
| D�� | Na��K�Ͻ��۵�ͣ�������ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| A�� | ��������������������Һ��Al+2OH-�TAlO2-+H2�� | |
| B�� | ����������̼ͨ��NaOH��Һ��CO2+OH-�THCO3- | |
| C�� | Cl2����ˮ��Cl2+H2O�T2H++Cl-+ClO- | |
| D�� | ���ˮ�еμӱ��͵��Ȼ�����Һ��Fe3++3H2O�TFe��OH��3��+3H+ |
| A�� | Al��Al2O3��Al��OH��3��AlCl3 | B�� | Fe��FeCl3��Fe��OH��3��Fe2O3 | ||
| C�� | Mg��MgCl2��Mg��OH��2��MgSO4 | D�� | Na��NaOH��Na2CO3��NaCl |
| A�� | ���� | B�� | FeCl3��Һ | C�� | ˮ | D�� | ��ˮ |

| A�� | ����ӦΪ���ȷ�Ӧ | |
| B�� | ͼ��P�㣺v��������v���棩 | |
| C�� | 950��ʱ��0��1.25s����H2��ƽ����Ӧ����Ϊ��0.016mol•L-1•s-1 | |
| D�� | 950��ʱ���÷�Ӧ��ƽ�ⳣ����ֵС��3.125��10-4 |
ʵ��һ����ȡ3.9200gĦ������Ʒ����250mL��Һ��
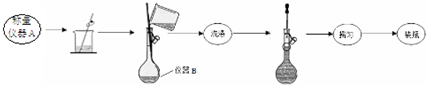
ʵ������ⶨĦ������Ʒ��ɵķ������£�
��ȡ��������Һ���������Ũ����������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����������ͺ��ɫ������
����ȡ��������Һ������KSCN��Һ������������
�۶����ⶨʵ�����£�
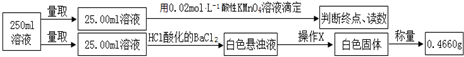
�ζ�ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ���/mL | 10.32 | 10.02 | 9.98 |
��1��ʵ��һ���������ƣ�����A������ƽ������B250mL����ƿ��
��2��д��ʵ��һ��ҡ�ȵ�ʵ�����������ƿƿ����������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ���µߵ�ҡ����Σ�ʹ��Һ��Ͼ��ȣ�
��3��ʵ������в���XΪ���ˡ�ϴ�ӡ�������ɣ���ȴ������������˳����д����
��4��ʵ������еζ�ʱ������Ӧ�����ӷ���ʽMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O���ζ��յ�����������һ����Һ���룬��Һ����dz�Ϻ�ɫ��30�벻��ɫ��
��5��ͨ������ʵ���ܷ�ȷ��Ħ������Ʒ������ܣ���ܡ��������ܡ���������
��д����ѧʽ����������˵�����ɣ�NH4��2SO4•FeSO4•6H2O��NH4��2Fe��SO4��2•6H2O��
 ����һ����Ҫ�Ĺ�ҵ�ܻ�������ϳ�·�ߺܶ࣬��ͼ�������е�һ�ֺϳɷ�����
����һ����Ҫ�Ĺ�ҵ�ܻ�������ϳ�·�ߺܶ࣬��ͼ�������е�һ�ֺϳɷ�����

 +2Cl2$\stackrel{����}{��}$
+2Cl2$\stackrel{����}{��}$ +2HCl��
+2HCl�� ��
��