��Ŀ����
17��ijѧϰС�鰴����ʵ�����̲ⶨĦ������Ʒ����ɣ�ʵ��һ����ȡ3.9200gĦ������Ʒ����250mL��Һ��
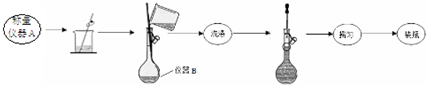
ʵ������ⶨĦ������Ʒ��ɵķ������£�
��ȡ��������Һ���������Ũ����������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����������ͺ��ɫ������
����ȡ��������Һ������KSCN��Һ������������
�۶����ⶨʵ�����£�
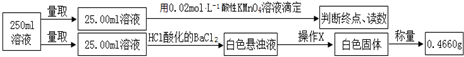
�ζ�ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ���/mL | 10.32 | 10.02 | 9.98 |
��1��ʵ��һ���������ƣ�����A������ƽ������B250mL����ƿ��
��2��д��ʵ��һ��ҡ�ȵ�ʵ�����������ƿƿ����������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ���µߵ�ҡ����Σ�ʹ��Һ��Ͼ��ȣ�
��3��ʵ������в���XΪ���ˡ�ϴ�ӡ�������ɣ���ȴ������������˳����д����
��4��ʵ������еζ�ʱ������Ӧ�����ӷ���ʽMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O���ζ��յ�����������һ����Һ���룬��Һ����dz�Ϻ�ɫ��30�벻��ɫ��
��5��ͨ������ʵ���ܷ�ȷ��Ħ������Ʒ������ܣ���ܡ��������ܡ���������
��д����ѧʽ����������˵�����ɣ�NH4��2SO4•FeSO4•6H2O��NH4��2Fe��SO4��2•6H2O��
���� ��1������Ħ������Һʱ����ȡ3.9200gĦ������ƷҪ�õ�����ƽ��ȡ������װ��ͼ��֪��������Һ����ʱҪ������ƿ��
��2��������ƿ��Һ��ҡ��Ҫ������ƿƿ����������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ���µߵ�ҡ����Σ�ʹ��Һ��Ͼ��ȣ��ݴ˴��⣻
��3������ʵ������в������̿�֪����ɫ����ҺΪ���ᱵ��Ҫ��ȷ�������ᱵ������Ҫ�������ˡ�ϴ�ӡ������ٳ�����
��4��ʵ������еζ�ʱ������ӦΪ����������������������ӣ��ζ��յ�ʱ��Һ�����dz�Ϻ�ɫ��Ϊ���������Һ����ɫ��
��5����������ʵ�鲽�輰���ݿ�֪���ζ�����������ȥ�ĸ��������Һ������������У���һ�����ϴ�ΪżȻ���Ӧȥ�������Ը��������Һ�����Ϊ$\frac{10.02+9.98}{2}$mL=10.00mL�����ݸ�����ص����ʵ�������ȷ���������ӵ����ʵ��������ݰ�ɫ����0.4660g����ȷ����������ӵ����ʵ��������õ���غ��֪笠����ӵ����ʵ���������Ʒ����������������ӡ��������Ӽ�笠����ӵ�����ȷ���ᾧˮ���������ݴ˿�ȷ��Ħ���ε���ɣ�
��� �⣺��1������Ħ������Һʱ����ȡ3.9200gĦ������ƷҪ�õ�����ƽ��ȡ������װ��ͼ��֪������250mL��Һ����ʱҪ��250mL����ƿ��
�ʴ�Ϊ��������ƽ��250mL����ƿ��
��2��������ƿ��Һ��ҡ��Ҫ������ƿƿ����������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ���µߵ�ҡ����Σ�ʹ��Һ��Ͼ��ȣ�
�ʴ�Ϊ��������ƿƿ����������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ���µߵ�ҡ����Σ�ʹ��Һ��Ͼ��ȣ�
��3������ʵ������в������̿�֪����ɫ����ҺΪ���ᱵ��Ҫ��ȷ�������ᱵ������Ҫ�������ˡ�ϴ�ӡ������ٳ�����
�ʴ�Ϊ�����ˡ�ϴ�ӡ�������ɣ���ȴ����
��4��ʵ������еζ�ʱ������ӦΪ����������������������ӣ���Ӧ�����ӷ���ʽΪMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O���ζ��յ�����������һ����Һ���룬��Һ����dz�Ϻ�ɫ��30�벻��ɫ��
�ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O�����һ����Һ���룬��Һ����dz�Ϻ�ɫ��30�벻��ɫ��
��5����������ʵ�鲽�輰���ݿ�֪���ζ�����������ȥ�ĸ��������Һ������������У���һ�����ϴ�ΪżȻ���Ӧȥ�������Ը��������Һ�����Ϊ$\frac{10.02+9.98}{2}$mL=10.00mL��������ص����ʵ���Ϊ0.02mol/L��0.01L=0.0002mol�����ݷ�ӦMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O����֪3.9200gĦ������Ʒ���������ӵ����ʵ���Ϊ0.0002mol��$\frac{250}{25}$��5=0.01mol��������Ϊ0.5600g����ɫ�������ᱵΪ0.4660g������3.9200gĦ������Ʒ����������ӵ����ʵ���Ϊ$\frac{0.4660}{233}$��$\frac{250}{25}$=0.02mol��������Ϊ1.9200g�����ݵ���غ��֪3.9200gĦ������Ʒ��笠����ӵ����ʵ���Ϊ0.02mol��2-0.01mol��2=0.02mol��������Ϊ0.3600g��������Ʒ�нᾧˮ�����ʵ���Ϊ$\frac{3.9200g-1.9200g-0.5600g-0.3600g}{18g/mol}$=0.06mol������Ħ���ε����Ϊ��NH4��2SO4•FeSO4•6H2O��NH4��2Fe��SO4��2•6H2O��
�ʴ�Ϊ���ܣ���NH4��2SO4•FeSO4•6H2O��NH4��2Fe��SO4��2•6H2O��
���� ���⿼�黯ѧ�������̡����ʵķ����ᴿ��������ԭ�ζ�Ӧ�á���Һ���Ƶȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ȷ����Ʒ���ʱע���غ�˼������ã��Ѷ��еȣ�

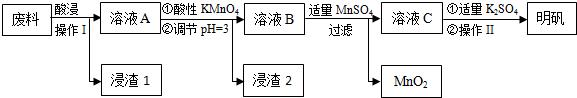
��1��������������е�Ӧѡ��H2SO4��д��Ļ�ѧʽ����Ϊ��߽������ʣ����ʵ��������Ũ���⣬���ɲ�ȡ�Ĵ�ʩ����߷�Ӧ�¶ȡ�����ȣ���д��������
��2���������ǹ��ˣ�������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��3������ҺA�м��������ط�����Ӧ�����ӷ���ʽΪ����������MnO4-ת��ΪMn2+��5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O��
��֪�������������������pH���±���ʾ
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
 ��
����4��ֻ��һ���Լ�������ҺA���Ƿ����Fe2+�����Լ��Ǹ������������Һ�����軯����Һ��д���ƣ���
��5����֪��pH=3�����������£�����MnSO4������Ӧ�����ӷ���ʽΪ3Mn2++2MnO4-+2H2O�T5MnO2+4H+��
| A�� | ���Ȼ�����Һ����Na2CO3��NaHCO3������Һ | |
| B�� | ������������Һ����MgCl2��Һ��AlCl3��Һ | |
| C�� | ���ö����ЧӦ����Fe��OH��3������FeCl3��Һ | |
| D�� | ����ɫ��Ӧ����NaCl��KCl��Na2SO4 |
| A�� | 0.46g | B�� | 0.69g | C�� | 0.92g | D�� | 0.23g |
| A�� | H2O2��H2O | B�� | NH4+��NH3 | C�� | Fe3+��Fe2+ | D�� | CO��CO2 |
| A�� | ��ʹ���ȱ�����Һ�У�Fe2+��Al3+��NO3-��Cl-��S2- | |
| B�� | ��pH=11����Һ�У�Na+��AlO2-��NO3-��S2-��SO32- | |
| C�� | �����£���ˮ�����c��H+��=10-10mol/L����Һ�У�Cl-��HCO3-��NO3-��NH4+��F- | |
| D�� | 0.1 mol•L-1 FeCl3��Һ�У�K+��Na+��AlO2-��SCN- |