��Ŀ����
6��Fe��Al�����ֳ��õĽ����������ǰ�һ������������ɻ�����1��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����Ϊn L����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����������е�Al�����ʵ���Ϊ$\frac{5n}{168}$�����ú���ĸ����ѧʽ��ʾ��
��2����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ$\frac{5m}{56}$g���������Fe������Ϊ2.5��m-n��g�����ú���ĸ����ѧʽ��ʾ��
��3����2�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������������������Ϊ30%��
���� ��1�������������Ʋ���Ӧ����������������Һ��Ӧ����ƫ�����ƺ�������������������֮��Ĺ�ϵʽ�����������ʵ�����
��2���÷�Ӧ��ϡ������������������������ת�Ƶ���֮��Ĺ�ϵʽ����ת�Ƶ��ӵ����ʵ�������ͬ��������������ϡ������������Ʒ�Ӧ���ɵ����������ͬ���ٸ�����������֮��Ĺ�ϵʽ��������������
��3����ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ�������������Ԫ�ص��������ڻ������Al���������Դ˼��㣮
��� �⣺��1�������������Ʋ���Ӧ����������������Һ��Ӧ����ƫ�����ƺ����������ӷ�Ӧ����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
���������ʵ���Ϊx��
2Al+2OH-+2H2O=2AlO2-+3H2��
2mol 67.2L
x nL
x=$\frac{2mol��nL}{67.2L}$=$\frac{5n}{168}$
�������Al�����ʵ���Ϊ$\frac{5n}{168}$��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����$\frac{5n}{168}$��
��2���÷�Ӧ��ת�Ƶ��������������ӵõ��ĵ�����=$\frac{m}{22.4}$��2=$\frac{5m}{56}$mol����ͬ��������������ϡ������������Ʒ�Ӧ���ɵ����������ͬ�����Ե�����������ϡ���ᷴӦ���������������nL��������ϡ���ᷴӦ�������������=��m-n��L��
������������y��
Fe+H2SO4=FeSO4+H2��
56g 22.4L
y ��m-n��L
y=$\frac{56g����m-n��L}{22.4L}$=2.5��m-n��g��
��ת�Ƶ��ӵ����ʵ�����$\frac{5m}{56}$������������2.5��m-n��g��
�ʴ�Ϊ��$\frac{5m}{56}$��2.5��m-n��g��
��3����2�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ�����������������������ԭ�������Al������������Al����������Ϊ$\frac{16��3}{160}$��100%=30%���ʴ�Ϊ��30%��
���� ���⿼����ۺϣ��漰�����Ļ�ѧ���ʣ����ӷ���ʽ����д�����㡢���������ļ���ȣ�ע����������������Һ��Ӧ��Fe���ܣ�����ѧ�����������Ŀ��飬��Ŀ�Ѷ��еȣ���3����ע���غ㷨��Ӧ��Ϊ���Ĺؼ���


| A�� | ����װ����ʯīI��ʯīII������ | |
| B�� | ��Ԫ����װ�â��б���������װ�â��б���ԭ | |
| C�� | ����MnO2�ĵ缫��ӦʽΪ��MnO2+2H2O+2e-�TMn2++4OH? | |
| D�� | ��Ӧ�١��������ɵ�����I2ʱ������ͨ���ĵ�����֮��Ϊ1��5 |
| A�� | ̼�������Һ������������������Һ��Ӧ��NH4++OH-=NH3•H2O | |
| B�� | ����ˮ��Ӧ��Na+2H2O=Na++2OH-+H2�� | |
| C�� | ͭ��ϡ���ᷴӦ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O | |
| D�� | �Ȼ�����Һ�����ԣ�Al3++3H2O?Al��OH��3��+3H + |
���ᡡ
�������
�ۺ����ᡡ
��һԪ�ᡡ
�ݵ����
����
���������
| A�� | �٢ڢۢܢݢ� | B�� | �٢ܢޢ� | C�� | �٢ڢۢܢ� | D�� | �٢ۢܢݢ� |
| A�� | Ne | B�� | F- | C�� | Na+ | D�� | K+ |
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
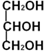 �����������ᣨCH3CH2COOH�����������ķ�Ӧ����ʽ��
�����������ᣨCH3CH2COOH�����������ķ�Ӧ����ʽ��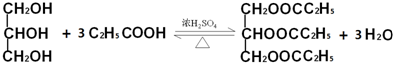
 �������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
�������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3�� ��
��