��Ŀ����
1�������й����ӹ����˵������ط�����ȷ���ǣ�������| ѡ�� | ������ | ���� |
| A | ������Al3+����Һ�У�K+��Na+��NO3-��CO32- | ���ܴ������棬����Al2��CO3��3�������� |
| B | ������Fe3+����Һ�У�K+��Mg2+��I-��NO3- | ���ܴ������棬��2Fe3++2I-=2Fe2++I2 |
| C | ��ˮ�����c��H+��=1��10-14mol/L����Һ�У� Ca2+��NO3-��HCO3-��Cl- | ���ܴ������棬����Һ�����ԣ���HCO3-��Ӧ����CO2���� |
| D | ʹ��̪������Һ�У� Na+��K+��SO32-��S2- | ���ܴ������棬��SO32-��S2-��Ӧ��������ɫ��S���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��Al3+��CO32-��������ˮ�ⷴӦ��
B��I-��Fe3+����������ԭ��Ӧ��
C����ˮ�����c��H+��=1��10-14mol/L����Һ���ܳ����Ի���ԣ�
D��ʹ��̪������Һ�ʼ��ԣ�
��� A��Al3+��CO32-��������ˮ�ⷴӦ����������������������A����
B��I-��Fe3+����������ԭ��Ӧ�����ӷ���ʽΪ2Fe3++2I-=2Fe2++I2����B��ȷ��
C����ˮ�����c��H+��=1��10-14mol/L����Һ���ܳ����Ի���ԣ����۳����Ի��Ǽ��ԣ�HCO3-�����ܴ������棬��C����
D��ʹ��̪������Һ�ʼ��ԣ���������������֮�䲻�����κη�Ӧ���ɴ������棬��D����
��ѡB��
���� ���⿼�����ӵĹ������⣬Ϊ�߿��������ͣ�������Ϣ������֮��ķ�ӦΪ���Ĺؼ������ظ��ֽⷴӦ��������ԭ��Ӧ�Ŀ��飬ѡ��AΪ�����״��㣬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
�����Ŀ
7�����ΪԪ�����ڱ��Ķ����ڲ��֣�
�����Ԫ��a-g�ڱ��е�λ�ã������жϳ���Ԫ�ػش����⣺
��1���Ƚ�d��eԪ�س������ӵİ뾶��С���û�ѧʽ��ʾ��O2-��Na+��
��2��b��c��Ԫ�طǽ����Խ�ǿ���ǣ�дԪ�ط��ţ�N��д��֤����һ���۵�һ����ѧ����ʽ2HNO3+Na2CO3 =2NaNO3+CO2��+H2O��
��3��d��eԪ���γɵ���ԭ�ӻ�����ĵ���ʽΪ ��b��gԪ���γɵ�bg2��Ϊ�ܼ����ܽ�S���ʣ���д�õ��ʡ����ơ���ѧʽ����
��b��gԪ���γɵ�bg2��Ϊ�ܼ����ܽ�S���ʣ���д�õ��ʡ����ơ���ѧʽ����
| a | |||||||
| b | c | d | |||||
| e | f | g |
��1���Ƚ�d��eԪ�س������ӵİ뾶��С���û�ѧʽ��ʾ��O2-��Na+��
��2��b��c��Ԫ�طǽ����Խ�ǿ���ǣ�дԪ�ط��ţ�N��д��֤����һ���۵�һ����ѧ����ʽ2HNO3+Na2CO3 =2NaNO3+CO2��+H2O��
��3��d��eԪ���γɵ���ԭ�ӻ�����ĵ���ʽΪ
 ��b��gԪ���γɵ�bg2��Ϊ�ܼ����ܽ�S���ʣ���д�õ��ʡ����ơ���ѧʽ����
��b��gԪ���γɵ�bg2��Ϊ�ܼ����ܽ�S���ʣ���д�õ��ʡ����ơ���ѧʽ����
12����Ӱ��ˮ�ĵ���ƽ�⣬����ʹˮ�������������Һ�б���Ϊc��OH-����c��H+���IJ����ǣ�������
| A�� | ��ˮ��Ͷ��һС������� | B�� | ��ˮ������� | ||
| C�� | ��ˮ��ͨ�������̼���� | D�� | ��ˮ�мӴ����ƾ��� |
16������ʵ��������Դﵽʵ��Ŀ���ǣ�������
| �� | ʵ ��Ŀ�� | ʵ����� |
| A�� | ��֤��Ȳ�ܱ����Ը��������Һ���� | ����ʯ�뱥��ʳ��ˮ��Ӧ���ɵ�����ֱ��ͨ�����Ը��������Һ���۲���Һ�Ƿ���ɫ |
| B�� | ��ȥ��ˮ�е������廯�� | �����Ҵ��������ã���Һ��ȡ���ϲ�Һ�� |
| C�� | ��ȥ���������л��е��������� | ������������Na2CO3��Һ�������ã���Һ��ȡ���ϲ�Һ�� |
| D�� | �����������е���Ԫ�� | ȡ���������飬������������Һ���Ⱥ������������Һ���۲��Ƿ���ֵ���ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
13��A��B��C����ǿ����ʣ�������ˮ�е���������������ʾ
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A��B��C������Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������C�缫����������54�ˣ������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ���£��ݴ˻ش���������
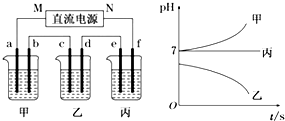
��1��NΪ��Դ������
��2������缫f�����ɵ������ڱ�״���µ����2.8L��
��3��д�����ձ��з�����Ӧ�Ļ�ѧ����ʽ4AgNO3+2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4HNO3��
��4�����������Һ�����Ϊ5 L�������Һ��pHΪ13��
��5��Ҫʹ���ָ���ԭ����״̬��Ӧ����4.5 gH2O������д��ѧʽ��
| ������ | Ag+��Na+ |
| ������ | NO3-��SO42-��Cl- |

��1��NΪ��Դ������
��2������缫f�����ɵ������ڱ�״���µ����2.8L��
��3��д�����ձ��з�����Ӧ�Ļ�ѧ����ʽ4AgNO3+2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4HNO3��
��4�����������Һ�����Ϊ5 L�������Һ��pHΪ13��
��5��Ҫʹ���ָ���ԭ����״̬��Ӧ����4.5 gH2O������д��ѧʽ��

 ��CCH2�TCHCN��
��CCH2�TCHCN�� ��ΪԪ�����ڱ���һ���֣������Ԫ�آ٢��ڱ��е�λ�ã��ش��������⣺
��ΪԪ�����ڱ���һ���֣������Ԫ�آ٢��ڱ��е�λ�ã��ش��������⣺