��Ŀ����
18�� ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺��1����Ũ������HCl�����ʵ���Ũ��Ϊ11.9 mol•L-1��
��2��ijѧ����������Ũ���������ˮ����500ml ���ʵ���Ũ��Ϊ0.400mol•L-1��ϡ���ᣮ
�ٸ�ѧ����Ҫ��ȡ16.8 ml ����Ũ����������ƣ�
�������ƹ����У�����ʵ����������Ƶ�ϡ��������ʵ���Ũ��ƫС����AB
A������Ͳ��ȡŨ��������ӹ۲찼Һ��
B�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ
C��������ƿ��ϴ����δ�����������ˮ���������Ƶ���ҺŨ�Ƚ�
D������ʱ���۾�����
E��δ��ȴ�����¾�ע������ƿ����
�����ƹ����У����õ����������ձ�������������Ͳ��轺ͷ�ιܡ�500mL����ƿ��
���� ��1������c=$\frac{1000�Ѧ�}{M}$�����Ũ������HCl�����ʵ���Ũ�ȣ�
��2���ٽ��ϡ�Ͷ��ɣ�c1v1=c2V2���㼴�ɣ�
������C=$\frac{n}{V}$����������ʹnƫС����Vƫ��IJ���������ʹ��Һ��Ũ��ƫС����֮��Ũ��ƫ��
�۸�������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��ʹ�õ�������
��� �⣺��1����������36.5%���ܶ�Ϊ1.19g/mL����������ʵ���Ũ��C=$\frac{1000��1.19��36.5%}{36.5}$=11.9mol/L��
�ʴ�Ϊ��11.9��
��2������ϡ�Ͷ��ɣ�c1v1=c2V2��֪������500mL0.4mol/L��ϡ���ᣬ���ø�Ũ����ΪC=$\frac{0.5��0.4}{11.9}$L=0.0168L=16.8mL��
�ʴ�Ϊ��16.8��
��A������Ͳ��ȡŨ��������ӹ۲찼Һ�棬��ȡ��Ũ�������ƫС��Ũ��ƫС����Aѡ��
B�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ����Һ�����ƫ��Ũ��ƫС����Bѡ��
C��������ƿ��ϴ����δ�����������ˮ�����ƹ�������Ҫ�μ�����ˮ���ݣ��ʴ���Ӱ�죬�ʢ۲�ѡ��
D������ʱ���۾�������Һ���ƫС��Ũ��ƫ��D��ѡ��
E��Һ���������������δ��ȴ�����¾�ע������ƿ���ݣ���Һ���ƫС��Ũ��ƫ��E��ѡ��
�ʴ�Ϊ��AB��
������500ml ���ʵ���Ũ��Ϊ0.400mol•L-1��ϡ���ᣬӦ��ѡ��500ml����ƿ������һ�����ʵ���Ũ�ȵ���Һ����Ϊ��������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ��һ������Ͳ��ȡŨ���ᣬ���ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ����Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ���ȱ�ٽ�ͷ�ιܡ�500mL����ƿ��
�ʴ�Ϊ����ͷ�ιܡ�500mL����ƿ��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ����Ŀ�ѶȲ�������������ѧ���ķ�����������ѧ����������

 ��У����ϵ�д�
��У����ϵ�д�| A�� |  ͼ�С�H1=��H2+��H3 | |
| B�� |  ��ͼ�ɿ���CO2����������CO+O2 | |
| C�� |  ��ͼ�ɿ�����Ӧ�м�������ɽ��ͷ�Ӧ������ | |
| D�� |  ͼ��t0ʱ��κ�����t0���������Һ�У�������ʷ��������Ӷ����� |
 ����ʱ����100mL0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ���õ�����ҺpHֵ��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��������Һ���ʱ������仯���������жϲ���ȷ���ǣ�������
����ʱ����100mL0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ���õ�����ҺpHֵ��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��������Һ���ʱ������仯���������жϲ���ȷ���ǣ�������| A�� | a����ʾ����Һ�У�c��Na+��=c��SO42-����c��NH4+�� | |
| B�� | b����ʾ����Һ�У�c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+�� | |
| C�� | c����ʾ����Һ�У�c��Na+��+c��NH4+��=2c��SO42-�� | |
| D�� | d����ʾ����Һ�У�c��NH4+��+10-9mol•L-1=$\frac{{K}_{w}}{1{0}^{-9}}$mol•L-1 |
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� �� 25�棩 | Ka=1.77��10-4 | Ka=5.0��10-10 | Ka1=4.3��10-7 Ka2=5.6��10-11 |
| A�� | ��NaCN��Һ��ͨ��������CO2���������ӷ�ӦΪ��2CN-+H2O+CO2�T2HCN+CO32- | |
| B�� | ������CN-��ˮʱ������NaOH��Һ����pH��9����ʱc��CN-����c��HCN�� | |
| C�� | �к͵��������pH��HCOOH��Һ��HCN��Һ����NaOH�����ʵ���ǰ��С�ں��� | |
| D�� | ������������ʵ���Ũ�ȵ�HCOONa��NaCN��Һ��������������ǰ�ߴ��ں��� |
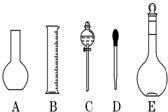 ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺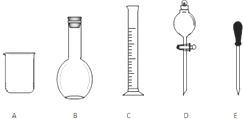 ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮
ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮ ����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ƹ����Ĺ������̣�
����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ƹ����Ĺ������̣�