��Ŀ����
3��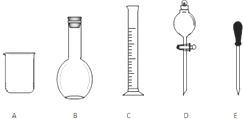 ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮
ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����BD������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������500mL����ƿ����������
��2��������ƿ��ʹ�÷����У����в�������ȷ����BC������ţ�
A��ʹ������ƿǰ�����Ƿ�©ˮ
B������NaOH��Һʱ���ѳƺõ�NaOH������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߣ�
C������H2SO4��Һʱ������ƿ������ˮϴ����Ҫ��0.5mol/L H2SO4��Һ��ϴ������ʹ�ã�
D���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ�
��3�����ݼ�����������ƽ��ȡ��NaOH��������Ϊ2.0g��
��4�����ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������
Ϊ13.6mL ������1λС������
��5���������ʹ�����Ƶ�ϡ����Ũ��ƫ����Ǣۣ�
������Ͳ��ȡŨ����ʱ���ӿ̶� ������ƿ������ϴ�Ӻ�δ����
��Ũ�����ܽ��δ��ȴ��ת�ơ����� �ܶ���ʱ���ӿ̶���
���� ��1�����ݸ�����������ѡȡ������
��2����������ƿ�Ĺ��켰��ȷʹ�÷������н��
��3������m=cVM�����������Ƶ���������ʵ������480mL��������ƿ��ע����Һ�����Ϊ500mL������480mL��
��4������c=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�������Һϡ��ǰ�����ʵ����ʵ������������ҪŨ����������
��5������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1������һ��Ũ�ȵ���Һ�����У�һ�������õ���ƿ�ͷ�Һ©������Ҫ��500mL����ƿ������Һ���ý�ͷ�ιܶ��ݣ�����������õ��Dz���������ȱ��500mL����ƿ�Ͳ�������
�ʴ�Ϊ��BD��500mL����ƿ����������
��2��A������ƿ��ƿ�������ƹ�������Ҫҡ�ȣ�Ϊ�˱���©Һ��ʹ������ƿǰ�����Ƿ�©ˮ����A��ȷ��
B������ƿΪ����������ֻ����������һ��Ũ�ȵ���Һ�����������ܽ����ϡ�����ʣ���B����
C������ƿ������ϴ������ᵼ�����Ƶ���Һ�����ʵ����ʵ���ƫ�����Ƶ���ҺŨ��ƫ�ߣ���C����
D�����ݽ�������Ҫ����ҡ�ȣ���������Ϊ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ���D��ȷ��
�ʴ�Ϊ��BC��
��3��0.1mol/LNaOH��Һ480mL����Ҫ����500mL 0.1mol/L����Һ����Ҫ�������Ƶ�����Ϊ��m=cVM=0.1mol/L��0.5L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��4����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������ʵ���Ũ��C=$\frac{1000��1.84g/ml��98%}{98g/mol}$=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=0.5/L��0.5L��V=0.0136L=13.6mL��
�ʴ�Ϊ��13.6��
��5��������Ͳ��ȡŨ����ʱ���ӿ̶��ߣ���ȡ��Ũ�������ƫС��Ũ��ƫ�ͣ��ʢٲ�ѡ��
������ƿ������ϴ�Ӻ�δ��������ƹ�������Ҫ�μ�����ˮ���ݣ��ʴ���Ӱ�죬�ʢڲ�ѡ��
��Ũ�����ܽ��δ��ȴ��ת�ơ����ݣ�δ��ȴ���ƫ���ݺ���ȴ���ƫС��Ũ��ƫ�ʢ�ѡ��
�ܶ���ʱ���ӿ̶��ߣ�����������Һ��Һ�����ƫ��Ũ��ƫ�ͣ��ʢܲ�ѡ��
��ѡ�ۣ�
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�ѶȲ�����ȷ���Ʋ���Ϊ���ؼ���ע���������ƹ������������ķ����뼼�ɣ���������������ѧ���Ļ�ѧʵ��������

| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g•cm-3�� | �ܽ��� |
| �� | -68 | 115 | 0.93 | ������ˮ |
| �� | -84 | 77 | 0.90 | �����ڼ� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | 4�� | B�� | 6�� | C�� | 8�� | D�� | 10�� |
| ��� | Cu | Zn | S |
| 1 | 10.3% | 5.0% | 1.2% |
| 2 | 11.5% | 4.9% | 1.8% |
| 3 | 12.4% | 10.3% | 0.9% |
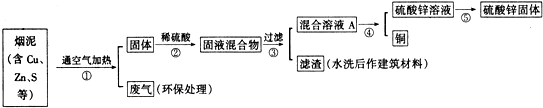
��1��д������٢��к�ͭԪ�ص����ʷ�����Ӧ�Ļ�ѧ����ʽ��2Cu+O2$\frac{\underline{\;����\;}}{\;}$2CuO��CuO+H2SO4=CuSO4+H2O��
��2��д���������д��������ķ������û�ѧ����ʽ��ʾ����2NaOH+SO2=Na2SO3+H2O��
��3������������õIJ��������ǣ������ᾧ����������Ũ�����ᾧ����
��4���ڲ�����У���ѡ���Լ�Zn�ӻ����ҺA�еõ�ͭ��
| A�� | 0.1mol/LCH3COOH��Һ����ˮϡ�����У���������Ũ�Ⱦ���С | |
| B�� | Ũ�Ⱦ�Ϊ0.1mol/L��NaF��CH3COONa��Һ��Ƚϣ�CH3COONa��ҺpH�� | |
| C�� | ��ӦHF+CH3COONa�TNaF+CH3COOH���Է��� | |
| D�� | NaF��Һ�м�����NaOH���壬��Һ��c��F-����� |
 25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��ΪCH3COOH��H2CO3��HClO��
��2��ͬŨ�ȵ�CH3COO-��HCO${\;}_{3}^{-}$��CO${\;}_{3}^{2-}$��ClO-���H+��������ǿ������˳��ΪCO32-��ClO-��HCO3-��CH3COO-��
��3�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1�������������ʵ���Һ��a��Na2CO3��b��NaClO��c��CH3COONa��d��NaHCO3��pH�ɴ�С��˳����a��b��d��c�����ţ���
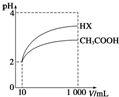
��4�����Ϊ10mL pH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ������pH�仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ��������ڡ���С�ڡ�������ĵ���ƽ�ⳣ����������ϡ����ͬ������HX��pH�仯��CH3COOH�Ĵ�����ǿ������ƽ�ⳣ����ϡ�ͺ�HX��Һ����ˮ���������c��H+�����ڣ�����ڡ��������ڡ���С�ڡ���������Һ����ˮ���������c��H+
����������HX����ǿ��CH3COOH�ģ�ϡ�ͺ�HX��Һ�е�c��H+��С��CH3COOH��Һ�е�c��H+�����������ˮ�������������Ҳ������
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺