��Ŀ����
5��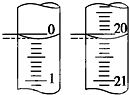 ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��������ɷֽ�Ϊ���¼�����
a����ȡ20.00mL�����NaOH��Һע��ྻ����ƿ��������2-3�η�̪
b���ñ�������Һ��ϴ�ζ���2-3��
c����ʢ�б���Һ����ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
d��ȡ��������Һע����ʽ�ζ�����0�̶�����2-3cm
e������Һ����0��0�̶����£����¶���
f������ƿ���ڵζ��ܵ����棬�ñ�������Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��bdceaf��
��2���ζ��յ�ʱ��Һ����ɫ�仯��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���D��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4����ij�εζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����Ӧ������������Ϊ20.00 mL��
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ����� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.20 | 20.22 | |
| �ڶ��� | 25.00 | 0.56 | 24.54 | |
| ������ | 25.00 | 0.42 | 20.40 | |
���� ��1�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�����
��2������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��3������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��4�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��5���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ���HCl+NaOH=NaCl+H2O���c��NaOH����
��� �⣺��1�������IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ�������˳��Ϊ��bdceaf��
�ʴ�Ϊ��bdceaf��
��2���ζ�ʱ��ƿ����Һ����ɫdz��ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ�����˵���ﵽ�ζ��յ㣻
�ʴ�Ϊ��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
��3��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ��Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩ƫ��A����
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬���V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩��Ӱ�죬��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩ƫ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩ƫ�ͣ���D��ȷ��
��ѡD��
��4����ʼ����Ϊ0.10mL���յ����Ϊ20.10mL��������Һ�����Ϊ20.00mL��
�ʴ�Ϊ��20.00��
��5�����εζ��������������ֱ�Ϊ��20.02mL��23.98mL��19.98mL���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=$\frac{19.98+20.02}{2}$mL=20mL��
���ݷ�Ӧ����ʽ��HCl+NaOH=NaCl+H2O��n��HCl��=n��NaOH��������0.02L��0.1000mol/L=0.025L��c��NaOH����
���c��NaOH��=0.0800mol/L��
�ʴ�Ϊ��0.0800mol/L��
���� ������Ҫ�������к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ���ǽ���ؼ���

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�| A�� | Al$\stackrel{����������}{��}$Al2O3$\stackrel{����}{��}$Al��NO3��3$\stackrel{��������}{��}$���������� | |
| B�� | Cu$\stackrel{����������}{��}$CuO$\stackrel{ϡ����}{��}$CuSO4$\stackrel{���ɽᾧ}{��}$���� | |
| C�� | Fe$\stackrel{����}{��}$FeCl3$\stackrel{������ˮ}{��}$Fe��OH��3$\stackrel{����}{��}$Fe2O3 | |
| D�� | FeSO4��Һ$\stackrel{���⡢����}{��}$FeS |
| ���� | ��1�� | ��2�� |
| ����ϡ���������/g | 50 | 50 |
| ʣ����������/g | 2.6 | 1 |
��1���Ͻ���þ������Ϊ4g��
��2������ϡ�������������������
 ij���Թ�ҵ��ˮ�к���K2Cr2O7�������£����ᣨH2C2O4���ܽ����е�Cr2O72-ת��ΪCr2+��ij�������о����֣�����������[Al2Fe��SO4��4•4H2O]���ɶԸ÷�Ӧ������ã�Ϊ��һ���о��й����ضԸ÷�Ӧ���ʵ�Ӱ�죬̽�����£�
ij���Թ�ҵ��ˮ�к���K2Cr2O7�������£����ᣨH2C2O4���ܽ����е�Cr2O72-ת��ΪCr2+��ij�������о����֣�����������[Al2Fe��SO4��4•4H2O]���ɶԸ÷�Ӧ������ã�Ϊ��һ���о��й����ضԸ÷�Ӧ���ʵ�Ӱ�죬̽�����£���1����25���£����ƹ���ǿ�ȡ���ˮ��Ʒ��ʼŨ�Ⱥʹ���������ͬ�����ڲ�ͬ�ij�ʼpH��һ��Ũ�Ȳ�����Һ���������Ա�ʵ�飬�������ʵ����Ʊ������в�Ҫ���ո�
| ʵ���� | ��ʼpH | ��ˮ��Ʒ���/mL | ������Һ���/mL | ����ˮ���/mL |
| ���� | 4 | a=60 | 10 | 30 |
| ���� | 5 | 60 | 10 | c=30 |
| ���� | 5 | 60 | b=20 | 20 |
��2��������Ӧ����ᱻ����ΪCO2���ѧʽ����
��3��ʵ��ٺ͢ڵĽ��������������������ʱ����Һ��pHԽС����Ӧ����Խ�죻
��4���ÿ������������[Al2Fe��SO4��4•4H2O]��������õijɷ�������¼��裬������ɼ�������
����һ��Fe2+������ã�
�������Al3+�������
��������SO42-������ã�
��5���������ʵ����֤��������һ������±������ݣ�����������ʵ���ṩ���Լ��⣬�ɹ�ѡ���ҩƷ��K2SO4��FeSO4��K2SO4•Al2��SO4��3•24H2O��Al2��SO4��3�ȣ�����Һ��Cr2O72-��Ũ�ȿ��������ⶨ��
| ʵ�鷽������Ҫ��д����������̣� | Ԥ��ʵ�����ͽ��� |
| ȡ�����ʵ�����K2SO4•Al2��SO4��3•24H2O����ʵ����е�������������������������ʵ�����ͬ�����жԱ�ʵ�飮 | ��Ӧ������ͬʱ��� ����Һ�е�Cr2O72-Ũ�ȴ��� ʵ����е�Cr2O72��Ũ�ȣ������һ������ ����Һ�е�Cr2O72-Ũ�ȵ��� ʵ����е�Cr2O72��Ũ�ȣ������һ�������� |
| ���� | 1 | 2 | 3 | 4 |
| ����V��KMnO4��/ml | 20.04 | 20.00 | 18.90 | 19.96 |
| A�� | ��c-a-b��kJ | B�� | ��a+b-c��kJ | C�� | ��2c-a-b��kJ | D�� | $\frac{2c-a-b}{2}$kJ |
| A�� | 2 | B�� | 3 | C�� | 4 | D�� | 5 |
 ��
��