��Ŀ����
14����ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�Ȼ�ѧ����ش��������⣺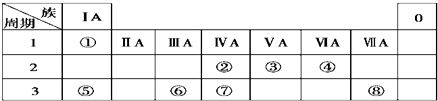
��1��Ԫ�آٵ��������⣮
��2��Ԫ�آڡ��ޡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳����Al��Si��C����Ԫ�ط��ű�ʾ����
��3��д��Ԫ�آ۵����������Ļ�ѧʽ��N2O5��
��4���õ���ʽ��ʾ��Ԫ�آ�����γɻ�����Ĺ��̣�
 ��
����5��Ԫ�آڵ����������+4������Ԫ�آܰ���ԭ�Ӹ�����Ϊ1��2�γɵĻ�����Ľṹʽ��O=C=O���û������ǹ��ۣ����ӡ����ۣ������
���� ��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1��Ԫ�آٵ��������⣻
��2��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��3��Ԫ�آ۵����������Ϊ������������
��4��������γɻ�����NaCl�����������������ӹ��ɣ�
��5������Ԫ����������ϼ۵���������������Ԫ�آ���Ԫ�آܰ���ԭ�Ӹ�����Ϊ1��2�γɵĻ�����ΪCO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1��Ԫ�آٵ��������⣬�ʴ�Ϊ���⣻
��2��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶Al��Si��C���ʴ�Ϊ��Al��Si��C��
��3��Ԫ�آ۵����������ΪN2O5���ʴ�Ϊ��N2O5��
��4��������γɻ�����NaCl�����������������ӹ��ɣ��õ���ʽ��ʾ�γɹ���Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��5������Ԫ����������ϼ۵���������������Ԫ�آڵ���������ϼ�Ϊ+4��Ԫ�آ���Ԫ�آܰ���ԭ�Ӹ�����Ϊ1��2�γɵĻ�����ΪCO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ��ṹʽΪO=C=O�����ڹ��ۻ����
�ʴ�Ϊ��+4��O=C=O�����ۣ�
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ������������ڱ��Ľṹ��ע���õ���ʽ��ʾ��ѧ�������ʵ��γɣ�

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�| A�� | ��������c��b | B�� | ���Ӱ뾶��X+��Z- | C�� | �ȶ��ԣ�H2Y��HZ | D�� | ���ԣ�XOH��W��OH��3 |
| X | Y | |||
| W |
| A�� | ����Ԫ���У�ԭ�Ӱ뾶������W | |
| B�� | Y �������Ա�W��������ǿ | |
| C�� | Y��Z�γɵĻ����ﶼ���Ժ�ˮ��Ӧ | |
| D�� | Z��Q�γɵĻ�����һ�������ӻ����� |
| X | ||
| Y | Z | W |
| T |
| A�� | X��W��ZԪ�ص���̬�⻯������ȶ��Ծ����ε��� | |
| B�� | Y��Z��WԪ������Ȼ���о�����������̬���ڣ����ǵ�����������ˮ������������ε��� | |
| C�� | YX2��WX3���������ӻ����� | |
| D�� | ����Ԫ�������ɣ������Ʋ�TԪ�صĵ��ʾ��а뵼�����ԣ�T2Y3���������Ժͻ�ԭ�� |
| A�� | ǿ����ʶ�������ˮ������BaSO4��������� | |
| B�� | һ�������´�����Һ�ĵ����������ܱ�ϡ����ǿ | |
| C�� | SO2��ˮ��Һ�ܵ��磬����SO2�ǵ���� | |
| D�� | ���ʯ�����磬��˽��ʯ�Ƿǵ���� |
�ټ�H2O��
�ڵ��뼸��ŨHNO3��
�۵��뼸��Ũ����
�ܼ�CH3COONa���塡
�ݼ�NaCl���塡
�������¶ȣ�����������ӷ�����
�߸���10mL 0.1mol•L-1���ᣮ
| A�� | �ڢޢ� | B�� | �ۢݢ� | C�� | �ۢޢ� | D�� | �ڢۢ� |
| A�� | NF3 | B�� | C${H}_{3}^{-}$ | C�� | SO3 | D�� | H3O+ |
| ������ | ������ | ���� | ���� | ������ |
| �ṹʽ | Cl-OH |  |  |  |
| ���ǻ���ԭ���� | 0 | 1 | 2 | 3 |
| ���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |
��H3PO3�ĽṹʽΪ
 ��H3PO3�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪH3PO3+2NaOH=Na2HPO3+2H2O��
��H3PO3�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪH3PO3+2NaOH=Na2HPO3+2H2O����H3AsO3�ĽṹʽΪ
 ����H3AsO3�м���Ũ���ᣬ��Ӧ�Ļ�ѧ����ʽΪAs��OH��3+3HCl=AsCl3+3H3O��
����H3AsO3�м���Ũ���ᣬ��Ӧ�Ļ�ѧ����ʽΪAs��OH��3+3HCl=AsCl3+3H3O�� 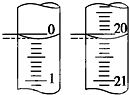 ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��������ɷֽ�Ϊ���¼�����
a����ȡ20.00mL�����NaOH��Һע��ྻ����ƿ��������2-3�η�̪
b���ñ�������Һ��ϴ�ζ���2-3��
c����ʢ�б���Һ����ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
d��ȡ��������Һע����ʽ�ζ�����0�̶�����2-3cm
e������Һ����0��0�̶����£����¶���
f������ƿ���ڵζ��ܵ����棬�ñ�������Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��bdceaf��
��2���ζ��յ�ʱ��Һ����ɫ�仯��dz��ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���D��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4����ij�εζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����Ӧ������������Ϊ20.00 mL��
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ����� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.20 | 20.22 | |
| �ڶ��� | 25.00 | 0.56 | 24.54 | |
| ������ | 25.00 | 0.42 | 20.40 | |