��Ŀ����
���ô�������Ϊ2-�һ�-1��3-�����������л���A������ԭ�Ϻϳ����ô�������X��·����ͼ1��

��֪��
��1��7.4g�л���A��ȫȼ�տɲ���0.4molCO2��0.5mol H2O��A����������������ܶ�Ϊ37���˴Ź���������ʾA����5��������ԭ�ӵ����շ壬�����֮��Ϊ3��3��2��1��1��
��2��
��X����±ԭ�ӣ�
��ش��������⣺
��1��A�ķ���ʽ�� ������������������ ��
��2���ڵĻ�ѧ��Ӧ������ ��F-G�ķ�Ӧ���� ��
��3���ٵĻ�ѧ��Ӧ����ʽ�� ��
��4��һ�������£���G����һ�ָ߷�����֬�Ļ�ѧ����ʽ�� ��
��5�������л���K�к�����Ԫ�ص�ʵ������� ��
��6��X�ж���ͬ���칹�壬����ͬʱ��������������ͬ���칹���� �֣���д������һ�ֵĽṹ��ʽ ��
�ٱ�����������ȡ��������������Ϊ��ͬ���ţ�
��1mol�л������2mol��������ˮ��Һ��ȫ��Ӧ�õ������л����

��֪��
��1��7.4g�л���A��ȫȼ�տɲ���0.4molCO2��0.5mol H2O��A����������������ܶ�Ϊ37���˴Ź���������ʾA����5��������ԭ�ӵ����շ壬�����֮��Ϊ3��3��2��1��1��
��2��

��X����±ԭ�ӣ�
��ش��������⣺
��1��A�ķ���ʽ��
��2���ڵĻ�ѧ��Ӧ������
��3���ٵĻ�ѧ��Ӧ����ʽ��
��4��һ�������£���G����һ�ָ߷�����֬�Ļ�ѧ����ʽ��
��5�������л���K�к�����Ԫ�ص�ʵ�������
��6��X�ж���ͬ���칹�壬����ͬʱ��������������ͬ���칹����
�ٱ�����������ȡ��������������Ϊ��ͬ���ţ�
��1mol�л������2mol��������ˮ��Һ��ȫ��Ӧ�õ������л����
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������A����������������ܶ�Ϊ37����A����Է�������Ϊ74��7.4g�л���A�����ʵ�����0.1mol����ȫȼ�տɲ���0.4molCO2��0.5mol H2O��A������C��Hԭ�Ӹ���֮��=0.4mol��1mol=2��5��0.4molCO2��0.5mol H2O��̼����Ԫ������=0.4mol��12g/mol+0.5mol��2��1g/mol=5.8g��7.4g������A�к���OԪ�أ�7.4gA��Oԭ�����ʵ���=
mol=0.1mol��
��A��C��H��Oԭ�Ӹ���֮��=0.4mol��1mol��0.1mol=4��10��1��
��A�ķ���ʽΪ��C4H10O��m������Է�������Ϊ74����m=
=1������A�ķ���ʽΪC4H10O���˴Ź���������ʾA����5��������ԭ�ӵ����շ壬�����֮��Ϊ3��3��2��1��1����A�Ľṹ��ʽΪCH3CH2CH��CH3��OH��A�ͱ����ᷢ��������Ӧ����X��X�ṹ��ʽΪ ��
��
���������Ϣ֪��A����������Ӧ����B��BΪ ���ɷ�Ӧ��Ϣ��֪��
���ɷ�Ӧ��Ϣ��֪�� ��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3��
��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3��
���ô�������Ϊ2-�һ�-1��3-���������ṹ��ʽΪHOCH2CH��C2H5��CH��OH��CH2CH2CH3�����E�����ô��Ľṹ��F�ķ���ʽ��֪��E�ڸ��»���������£�C=C�м���Hԭ�ӱ�Brԭ��ȡ������FΪCH3CH2C��CH2Br��=CHCH2CH2CH3��F����������ˮ��Һ�����������·���ˮ�ⷴӦ����G��G�Ľṹ��ʽΪCH3CH2C��CH2OH��=CHCH2CH2CH3��G�����ӳɷ�Ӧ����K��KΪCH3CH2C��CH2Br��CH��Br��CH2CH2CH3��K��������ˮ��Һ�����������·���ˮ�ⷴӦ�������ô����ݴ˷������
| 7.4-5.8 |
| 16 |
��A��C��H��Oԭ�Ӹ���֮��=0.4mol��1mol��0.1mol=4��10��1��
��A�ķ���ʽΪ��C4H10O��m������Է�������Ϊ74����m=
| 74 |
| 12��4+1��10+16��1 |
 ��
�����������Ϣ֪��A����������Ӧ����B��BΪ
 ���ɷ�Ӧ��Ϣ��֪��
���ɷ�Ӧ��Ϣ��֪�� ��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3��
��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3�����ô�������Ϊ2-�һ�-1��3-���������ṹ��ʽΪHOCH2CH��C2H5��CH��OH��CH2CH2CH3�����E�����ô��Ľṹ��F�ķ���ʽ��֪��E�ڸ��»���������£�C=C�м���Hԭ�ӱ�Brԭ��ȡ������FΪCH3CH2C��CH2Br��=CHCH2CH2CH3��F����������ˮ��Һ�����������·���ˮ�ⷴӦ����G��G�Ľṹ��ʽΪCH3CH2C��CH2OH��=CHCH2CH2CH3��G�����ӳɷ�Ӧ����K��KΪCH3CH2C��CH2Br��CH��Br��CH2CH2CH3��K��������ˮ��Һ�����������·���ˮ�ⷴӦ�������ô����ݴ˷������
���
�⣺A����������������ܶ�Ϊ37����A����Է�������Ϊ74��7.4g�л���A�����ʵ�����0.1mol����ȫȼ�տɲ���0.4molCO2��0.5mol H2O��A������C��Hԭ�Ӹ���֮��=0.4mol��1mol=2��5��0.4molCO2��0.5mol H2O��̼����Ԫ������=0.4mol��12g/mol+0.5mol��2��1g/mol=5.8g��7.4g������A�к���OԪ�أ�7.4gA��Oԭ�����ʵ���=
mol=0.1mol��
��A��C��H��Oԭ�Ӹ���֮��=0.4mol��1mol��0.1mol=4��10��1��
��A�ķ���ʽΪ��C4H10O��m������Է�������Ϊ74����m=
=1������A�ķ���ʽΪC4H10O���˴Ź���������ʾA����5��������ԭ�ӵ����շ壬�����֮��Ϊ3��3��2��1��1����A�Ľṹ��ʽΪCH3CH2CH��CH3��OH��A�ͱ����ᷢ��������Ӧ����X��X�ṹ��ʽΪ ��
��
���������Ϣ֪��A����������Ӧ����B��BΪ ���ɷ�Ӧ��Ϣ��֪��
���ɷ�Ӧ��Ϣ��֪�� ��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3��
��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3��
���ô�������Ϊ2-�һ�-1��3-���������ṹ��ʽΪHOCH2CH��C2H5��CH��OH��CH2CH2CH3�����E�����ô��Ľṹ��F�ķ���ʽ��֪��E�ڸ��»���������£�C=C�м���Hԭ�ӱ�Brԭ��ȡ������FΪCH3CH2C��CH2Br��=CHCH2CH2CH3��F����������ˮ��Һ�����������·���ˮ�ⷴӦ����G��G�Ľṹ��ʽΪCH3CH2C��CH2OH��=CHCH2CH2CH3��G�����ӳɷ�Ӧ����K��KΪCH3CH2C��CH2Br��CH��Br��CH2CH2CH3��K��������ˮ��Һ�����������·���ˮ�ⷴӦ�������ô���
��1��ͨ�����Ϸ���֪��A�ķ���ʽΪC4H10O���ṹ��ʽΪCH3CH2CH��CH3��OH��A�й��������ǻ���
�ʴ�Ϊ��C4H10O���ǻ���
��2��ͨ�����Ϸ���֪�����Ƿ�����ȥ��Ӧ����Ӧ������Ũ���ᡢ���ȣ�F��NaOH��ˮ��Һ����ȡ����Ӧ����G�����Է�Ӧ������ȡ����Ӧ��
�ʴ�Ϊ��Ũ���ᡢ���ȣ�ȡ����Ӧ��
��3����Ӧ���� �뱽���ᷢ��������Ӧ����
�뱽���ᷢ��������Ӧ���� ����Ӧ����ʽΪ��
����Ӧ����ʽΪ�� +
+
 +H2O��
+H2O��
�ʴ�Ϊ�� +
+
 +H2O��
+H2O��
��4��һ�������£�CH3CH2C��CH2OH��=CHCH2CH2CH3�����Ӿ۷�Ӧ����һ�ָ߷�����֬����Ӧ����ʽΪ��
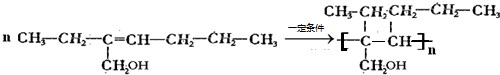 ��
��
�ʴ�Ϊ��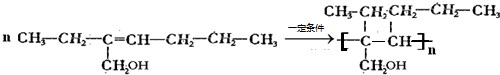 ��
��
��5��Ҫ����K�к�����Ԫ�أ�Ӧ���Ƚ���Ԫ��ת��Ϊ�����ӣ������������ữ����������Һ���������ӣ�������ɵ���ɫ��������˵��K�к�����Ԫ�أ�����鷽����ȡ�����л���K�������Թ��У���������������Һ���ȣ�һ��ʱ���ȡ���ϲ���Һ����ϡ�����ữ���ټ��������������е���ɫ�������ɣ�֤������Ԫ�أ�
�ʴ�Ϊ��ȡ�����л���K�������Թ��У���������������Һ���ȣ�һ��ʱ���ȡ���ϲ���Һ����ϡ�����ữ���ټ��������������е���ɫ�������ɣ�֤������Ԫ�أ�
��6��XΪ ����ͬ���칹����������Ϊ��ͬ���ţ���1mol�л������2mol��������ˮ��Һ��ȫ��Ӧ�õ������л������ȡ����Ϊ-OOCCH2CH3��2��-CH3��-OOCH��2��-CH2CH3����2��-CH3���ڣ�-OOCCH2CH3��2��λ�ù�ϵ����2��-CH3��䣬-OOCCH2CH3��3��λ�ù�ϵ����2��-CH3��ԣ�-OOCCH2CH3��1��λ�ù�ϵ������6��ͬ���칹�壬ͬ��ȡ����Ϊ-OOCH��2��-CH2CH3����6��ͬ���칹�壬���Է���������ͬ���칹����12�֣�����һ��Ϊ
����ͬ���칹����������Ϊ��ͬ���ţ���1mol�л������2mol��������ˮ��Һ��ȫ��Ӧ�õ������л������ȡ����Ϊ-OOCCH2CH3��2��-CH3��-OOCH��2��-CH2CH3����2��-CH3���ڣ�-OOCCH2CH3��2��λ�ù�ϵ����2��-CH3��䣬-OOCCH2CH3��3��λ�ù�ϵ����2��-CH3��ԣ�-OOCCH2CH3��1��λ�ù�ϵ������6��ͬ���칹�壬ͬ��ȡ����Ϊ-OOCH��2��-CH2CH3����6��ͬ���칹�壬���Է���������ͬ���칹����12�֣�����һ��Ϊ �ȣ�
�ȣ�
�ʴ�Ϊ��12�� ��
��
| 7.4-5.8 |
| 16 |
��A��C��H��Oԭ�Ӹ���֮��=0.4mol��1mol��0.1mol=4��10��1��
��A�ķ���ʽΪ��C4H10O��m������Է�������Ϊ74����m=
| 74 |
| 12��4+1��10+16��1 |
 ��
�����������Ϣ֪��A����������Ӧ����B��BΪ
 ���ɷ�Ӧ��Ϣ��֪��
���ɷ�Ӧ��Ϣ��֪�� ��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3��
��CH3CH2CH2CH2MgCl�����ӳɷ�Ӧ����C��C��ˮ������D����DΪCH3C��OH����C2H5��CH2CH2CH2CH3��D������ȥ��Ӧ����E��3-��-3-��ϩ����E�ṹ��ʽΪCH3CH2C��CH3��=CHCH2CH2CH3�����ô�������Ϊ2-�һ�-1��3-���������ṹ��ʽΪHOCH2CH��C2H5��CH��OH��CH2CH2CH3�����E�����ô��Ľṹ��F�ķ���ʽ��֪��E�ڸ��»���������£�C=C�м���Hԭ�ӱ�Brԭ��ȡ������FΪCH3CH2C��CH2Br��=CHCH2CH2CH3��F����������ˮ��Һ�����������·���ˮ�ⷴӦ����G��G�Ľṹ��ʽΪCH3CH2C��CH2OH��=CHCH2CH2CH3��G�����ӳɷ�Ӧ����K��KΪCH3CH2C��CH2Br��CH��Br��CH2CH2CH3��K��������ˮ��Һ�����������·���ˮ�ⷴӦ�������ô���
��1��ͨ�����Ϸ���֪��A�ķ���ʽΪC4H10O���ṹ��ʽΪCH3CH2CH��CH3��OH��A�й��������ǻ���
�ʴ�Ϊ��C4H10O���ǻ���
��2��ͨ�����Ϸ���֪�����Ƿ�����ȥ��Ӧ����Ӧ������Ũ���ᡢ���ȣ�F��NaOH��ˮ��Һ����ȡ����Ӧ����G�����Է�Ӧ������ȡ����Ӧ��
�ʴ�Ϊ��Ũ���ᡢ���ȣ�ȡ����Ӧ��
��3����Ӧ����
 �뱽���ᷢ��������Ӧ����
�뱽���ᷢ��������Ӧ���� ����Ӧ����ʽΪ��
����Ӧ����ʽΪ�� +
+
| ŨH2SO4 |
| �� |
 +H2O��
+H2O���ʴ�Ϊ��
 +
+
| ŨH2SO4 |
| �� |
 +H2O��
+H2O����4��һ�������£�CH3CH2C��CH2OH��=CHCH2CH2CH3�����Ӿ۷�Ӧ����һ�ָ߷�����֬����Ӧ����ʽΪ��
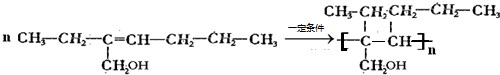 ��
���ʴ�Ϊ��
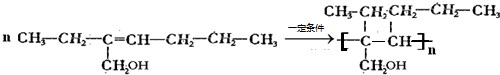 ��
����5��Ҫ����K�к�����Ԫ�أ�Ӧ���Ƚ���Ԫ��ת��Ϊ�����ӣ������������ữ����������Һ���������ӣ�������ɵ���ɫ��������˵��K�к�����Ԫ�أ�����鷽����ȡ�����л���K�������Թ��У���������������Һ���ȣ�һ��ʱ���ȡ���ϲ���Һ����ϡ�����ữ���ټ��������������е���ɫ�������ɣ�֤������Ԫ�أ�
�ʴ�Ϊ��ȡ�����л���K�������Թ��У���������������Һ���ȣ�һ��ʱ���ȡ���ϲ���Һ����ϡ�����ữ���ټ��������������е���ɫ�������ɣ�֤������Ԫ�أ�
��6��XΪ
 ����ͬ���칹����������Ϊ��ͬ���ţ���1mol�л������2mol��������ˮ��Һ��ȫ��Ӧ�õ������л������ȡ����Ϊ-OOCCH2CH3��2��-CH3��-OOCH��2��-CH2CH3����2��-CH3���ڣ�-OOCCH2CH3��2��λ�ù�ϵ����2��-CH3��䣬-OOCCH2CH3��3��λ�ù�ϵ����2��-CH3��ԣ�-OOCCH2CH3��1��λ�ù�ϵ������6��ͬ���칹�壬ͬ��ȡ����Ϊ-OOCH��2��-CH2CH3����6��ͬ���칹�壬���Է���������ͬ���칹����12�֣�����һ��Ϊ
����ͬ���칹����������Ϊ��ͬ���ţ���1mol�л������2mol��������ˮ��Һ��ȫ��Ӧ�õ������л������ȡ����Ϊ-OOCCH2CH3��2��-CH3��-OOCH��2��-CH2CH3����2��-CH3���ڣ�-OOCCH2CH3��2��λ�ù�ϵ����2��-CH3��䣬-OOCCH2CH3��3��λ�ù�ϵ����2��-CH3��ԣ�-OOCCH2CH3��1��λ�ù�ϵ������6��ͬ���칹�壬ͬ��ȡ����Ϊ-OOCH��2��-CH2CH3����6��ͬ���칹�壬���Է���������ͬ���칹����12�֣�����һ��Ϊ �ȣ�
�ȣ��ʴ�Ϊ��12��
 ��
��
���������⿼���л����ƶϣ�Ϊ�߿���Ƶ�㣬��ȷ�ƶ�A�ṹ�ǽⱾ��ؼ����ٽ�������Ϣ����Ӧ����������ͼ�з���ʽ�����ƶϣ�֪�������л������ʼ���ɼ��Ͷϼ���ʽ��ע�⣨5���еμ���������Һ֮ǰҪ�������кͼΪ�״��㣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A���ܻ���DEHP��ָ�ڱ����������2���һ�������������������������ʳƷ���Ӽ� |
| B����������������ѹ������ɫҺ��Ϊ�����仯 |
| C��Na2O?SiO2��һ�ּĹ����Σ�������ˮ |
| D��Ϊ�ⶨ�����������Ƶĵ����ԣ��ɽ��������ƹ������ʯӢ�����м����ۻ� |
����˵������ȷ���ǣ�������
| A�����ۺ���ά�ؾ�Ϊ�߷��ӻ�������߶��ǹ�ҵ����ƾ���ԭ�� |
| B��CH2=CH-CH��CH3��-C��CH�������������3-������ |
| C���������ļ��顢�Ҵ�����ȩ�ֱ���ȼ�գ������������������μ��� |
D����֪ƻ����Ľṹ��ʽΪ ��������ʿɷ���������Ӧ��������Ӧ�����۷�Ӧ����HOOC-CH2-CH��OH��-COOH��Ϊͬ���칹�� ��������ʿɷ���������Ӧ��������Ӧ�����۷�Ӧ����HOOC-CH2-CH��OH��-COOH��Ϊͬ���칹�� |
��100mL�ܶ�Ϊ1.2g/mLϡ�����У�����һ������þ��ͭ��ɵĻ�����ַ�Ӧ�������ȫ�ܽ⣨���軹ԭ����ֻ��NO������Ӧ����Һ�м���3mol/L NaOH��Һ��������ȫ��������ɳ���������ԭ������������5.1g����������������ȷ�ǣ�������
| A��������ȫ���ܽ�ʱ�ռ���NO��������Ϊ2.24L����״���� |
| B�������ɳ����������ʱ������NaOH��Һ�����СΪ100mL |
| C��ԭϡ��������ʵ���Ũ��һ��Ϊ4mol/L |
| D���μӷ�Ӧ������������m��Ϊ9.6g��m��3.6g |
����ѪҺ�д��ڵ�ƽ�⣺H2CO3?H++HCO3-��ʹѪҺpH������7.35��7.45֮�䣬����ͻᷢ�����ж�����ж�����֪pH�� �仯��ϵ�����ʾ��������˵���д�����ǣ�������
| 1.0 | 17.8 | 20.0 | 22.4 | ||||
| pH | 6.10 | 7.35 | 7.40 | 7.45 |
| A��pH=7��ѪҺ�У�c��HCO3-����c��H2CO3�� | ||||
| B���������������巢�����ж�ʱ��c��H+��?c��OH-����� | ||||
| C�����巢�����ж�ʱ���ɾ�����עһ��Ũ�ȵ�NaHCO3��Һ�ⶾ | ||||
D��
|


