��Ŀ����
6��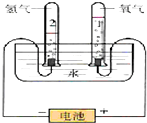 ˮ�����౦�����Ȼ��Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ���ͼ�ǵ��ˮԭ����ʵ��װ��ͼ��
ˮ�����౦�����Ȼ��Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ���ͼ�ǵ��ˮԭ����ʵ��װ��ͼ����1��д�����ˮ�Ļ�ѧ����ʽ����Ϊ������ԭ��Ӧ������˫���ű�ʾ�����ӵ�ת�Ʒ������Ŀ��
 ��
����2����������3.6gH2O��������±���
| �� �� | ���ʵ��� | O2��H2������ȣ�ͬ��ͬѹ�£� | |
| O2 | 3.2g | 0.1mol | 1��2 |
| H2 | 0.4g | 0.2mol |
��4�����ڵ��ˮ���ռ���O2���Ϊ22.4mL���ڱ�״���£������ռ�����H2������0.004g��
���� ��1��������Ӧ��2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2����ת�Ƶ�����ĿΪ4e-��
��2������n=$\frac{m}{M}$����ˮ�����ʵ���������2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2�����㣻
��3��������Ӧ��2Na+2 H2O�T2NaOH+H2������Ӧ����Һ������ΪNaOH�����ݷų���������NaOH��������������Һ����=ˮ������+�Ƶ�����-��������������������Һ��NaOH��������������n=$\frac{m}{M}$���Եõ�NaOH���ʵ�����Ҫ�������Һ�����ʵ����ʵ���Ũ�ȣ���Ҫ��Һ��������ڿ��Ի����Һ����������Ҫ��Һ�ܶȣ�
��4������n=$\frac{V}{{V}_{m}}$�����������ʵ��������ݷ���ʽ���������������ʵ������ٸ���m=nVm��������������
��� �⣺��1��������Ӧ��2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2����ת�Ƶ�����ĿΪ4e-����˫���ű�ʾ�����ӵ�ת�Ʒ������ĿΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��3.6gH2O�����ʵ���Ϊ$\frac{3.6g}{18g/mol}$=0.2mol��
2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2��
2 2 1
0.2mol 0.2mol 0.1mol
��������������Ϊ0.1mol��32g/mol=3.2g��ʧȥ��������Ϊ0.2mol��2g/mol=0.4g��
ͬ��ͬѹ��O2��H2�������Ϊ0.1mol��0.2mol=1��2��
�ʴ�Ϊ��
| �� �� | ���ʵ��� | O2��H2������ȣ�ͬ��ͬѹ�£� | |
| O2 | 3.2g | 0.1mol | 1��2 |
| H2 | 0.4g | 0.2mol |
��3��������Ӧ��2Na+2 H2O�T2NaOH+H2������Ӧ���ӷ���ʽΪ��2Na+2 H2O�T2Na++2OH-+H2������Ӧ����Һ������ΪNaOH��
2Na+2 H2O�T2NaOH+H2��
46 80 2
2.3g x y
��x=$\frac{2.3g��80}{46}$=4g
y=$\frac{2.3g��2}{46}$=0.1g
��Ӧ����Һ����Ϊ2.3g+47.8g-0.1g=50g��
����Һ��NaOH��������Ϊ$\frac{4g}{50g}$��100%=8%��
����n=$\frac{m}{M}$���Եõ�NaOH���ʵ�����Ҫ�������Һ�����ʵ����ʵ���Ũ�ȣ���Ҫ��Һ��������ڿ��Ի����Һ����������Ҫ��Һ�ܶȣ�
�ʴ�Ϊ��2Na+2 H2O�T2Na++2OH-+H2����NaOH��80%����Һ�ܶȣ�
��4���������ʵ���Ϊ$\frac{0.0224L}{22.4L/mol}$=0.001mol����2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2������֪��������Ϊ0.001mol��2=0.002mol����������������Ϊ0.002mol��2g/mol=0.004g��
�ʴ�Ϊ��0.004g��
���� ���⿼�黯ѧ����ʽ���㡢������ԭ��Ӧ����ҺŨ���йؼ���ȣ�ע�������õ����š�˫���ű�ʾ����ת����Ŀ�뷽��

| A�� | NH3��CuS��SO2��FeS | B�� | SO3��Cu2S��FeI2��FeCl3 | ||
| C�� | FeCl3��SO2��NO��Cu2S | D�� | FeCl2��FeS��SO2��FeI3 |
| A�� | �ɱ�-H2O | B�� | ˮ��-Ag | C�� | �ƾ�-C2H5OH | D�� | ����-NaOH |
| ѡ�� | ����I | ����II |
| A | п������Ա���ǿ | ���������װп��ɼ�����ʴ |
| B | Ba��OH��2�������ᷴӦ | Ba��OH��2����������θ����� |
| C | SiO2������������ | SiO2����ˮ��Ӧ���ɹ��� |
| D | H2O2�������� | H2O2��ʹ���Ը��������Һ��ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | z=2 | B�� | 2s�������ڵ�ѹǿ�dz�ʼ��$\frac{7}{8}$�� | ||
| C�� | 2sʱC���������Ϊ$\frac{2}{7}$ | D�� | 2s��B��Ũ��Ϊ0.5mol/L |
| A�� | �÷�Ӧ��Na2Sx����������NaClO�ǻ�ԭ�� | |
| B�� | Na2Sx�������Ӽ��ͼ��Թ��ۼ� | |
| C�� | 1molNa2Sx�μӷ�Ӧ����32mol����ת�� | |
| D�� | Na2Sx�е�X��ֵΪ2 |
| A�� | 46 g��NO2��N2O4������庬�е�ԭ����Ϊ3NA | |
| B�� | 1 mol Na2O2������H2O��Ӧ��ת�Ƶĵ�����ΪNA | |
| C�� | ���ʵ���Ũ��Ϊ0.5 mol/L MgCl2��Һ������Cl-������ΪNA | |
| D�� | 2.7g�������������������������Һ��Ӧ������H2�ķ�������Ϊ0.15NA |
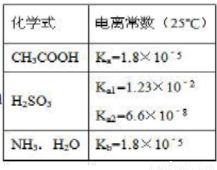
 OOH) =c (CH3COO-
OOH) =c (CH3COO- ) +2c (OH-)
) +2c (OH-)