��Ŀ����
��1��ijѧ����ʵ�鱨����������ʵ�����ݣ��������ݺ������� ��
A����������ƽ��ȡ11.7g�Ȼ���
B������Ͳ��ȡ5.26mL����
C���ù㷺pH��ֽ�����Һ��pH��3.5
D���к͵ζ�ʱ��ȥ23.10mLNaOH��Һ
��2����һѧ����ʵ���Ҳ�ij��ҺpH��ʵ��ʱ������������ˮ��ʪpH��ֽ��Ȼ���ýྻ����IJ�����պȡ�������м�⣮
���ִ������ ���һ����/��һ������/����һ�������ᵼ��ʵ��������
�����˷��ֱ�ⶨc��H+����ȵ�����ʹ�����Һ��pH�����ϴ���� ��
�����к͵ζ���ȷ��ij�ռ���Ʒ��Ũ�ȣ��Ը���ʵ��ش��������⣺
��1��ȷ��ȡһ��������Ʒ�����500mL������Һ������ʱ����Ʒ�ɷ��� ��������ĸ���ϳ���
A С�ձ� B �ྻֽƬ C ֱ�ӷ���������
��2���ζ�ʱ����0.2000mol?L-1���������ζ�������Һ������ѡ�� ��������ĸ����ָʾ������
A ���� B ʯ�� C ��̪
��3����ѡ�÷�̪��ָʾ�����ζ��յ���ж������� ��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ���� mol?L-1��
��5������ʵ�������Եζ���������ĺ�������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ���� ��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ���� ��
A����������ƽ��ȡ11.7g�Ȼ���
B������Ͳ��ȡ5.26mL����
C���ù㷺pH��ֽ�����Һ��pH��3.5
D���к͵ζ�ʱ��ȥ23.10mLNaOH��Һ
��2����һѧ����ʵ���Ҳ�ij��ҺpH��ʵ��ʱ������������ˮ��ʪpH��ֽ��Ȼ���ýྻ����IJ�����պȡ�������м�⣮
���ִ������
�����˷��ֱ�ⶨc��H+����ȵ�����ʹ�����Һ��pH�����ϴ����
�����к͵ζ���ȷ��ij�ռ���Ʒ��Ũ�ȣ��Ը���ʵ��ش��������⣺
��1��ȷ��ȡһ��������Ʒ�����500mL������Һ������ʱ����Ʒ�ɷ���
A С�ձ� B �ྻֽƬ C ֱ�ӷ���������
��2���ζ�ʱ����0.2000mol?L-1���������ζ�������Һ������ѡ��
A ���� B ʯ�� C ��̪
��3����ѡ�÷�̪��ָʾ�����ζ��յ���ж�������
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����
| �ζ����� | ������Һ�����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.20 | 24.00 |
| ������ | 10.00 | 0.40 | 21.50 |
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����
���㣺�к͵ζ�,̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������1��A��������ƽ�ĸ�����0.1g��
B����Ͳ����С�̶���0.1mL��
C���㷺pH��ֽ�IJ���ֵ��������
D���ζ��ܵĸ�����0.01mL��
��2��������ˮ��ʪ��ֽ�ᵼ����ҺŨ�Ƚ��ͣ�����Һ��pH��һ���ı䣻��ˮϡ�ʹٽ�������ʵ��룬��ͬŨ�ȵ�һԪǿ����ϡ����ͬ�ı�����������������Ũ�ȴ���ǿ�
��1��NaOH���и�ʴ�ԣ�Ӧ�÷���С�ձ��г�����
��2���ζ�ʱ����0.2000mol?L-1���������ζ�������Һ��Ӧ��ѡ�ü��Ȼ��̪��ָʾ����
��3���ζ��յ��жϷ����ǣ����������һ�α�����Һʱ����Һ�պ��ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ɫ��
��4���ζ�������ʹ�����ƽ�����=
mL=20.00mL��
�������Ǽ��2�����������ʵ���Ũ�������2����
��5��������ʱ��Һ���ƫС�����²ⶨ��ҺŨ��ƫ�ͣ�
��������ƿ�ô���Һ��ϴ���ᵼ������������ƫ�ⶨ��ҺŨ��ƫ�ߣ�
B����Ͳ����С�̶���0.1mL��
C���㷺pH��ֽ�IJ���ֵ��������
D���ζ��ܵĸ�����0.01mL��
��2��������ˮ��ʪ��ֽ�ᵼ����ҺŨ�Ƚ��ͣ�����Һ��pH��һ���ı䣻��ˮϡ�ʹٽ�������ʵ��룬��ͬŨ�ȵ�һԪǿ����ϡ����ͬ�ı�����������������Ũ�ȴ���ǿ�
��1��NaOH���и�ʴ�ԣ�Ӧ�÷���С�ձ��г�����
��2���ζ�ʱ����0.2000mol?L-1���������ζ�������Һ��Ӧ��ѡ�ü��Ȼ��̪��ָʾ����
��3���ζ��յ��жϷ����ǣ����������һ�α�����Һʱ����Һ�պ��ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ɫ��
��4���ζ�������ʹ�����ƽ�����=
| [(20.50-0.40)+(24.00-4.20)+(21.50-0.40)] |
| 3 |
�������Ǽ��2�����������ʵ���Ũ�������2����
��5��������ʱ��Һ���ƫС�����²ⶨ��ҺŨ��ƫ�ͣ�
��������ƿ�ô���Һ��ϴ���ᵼ������������ƫ�ⶨ��ҺŨ��ƫ�ߣ�
���
�⣺��1��A��������ƽ�ĸ�����0.1g��������������ƽ���Գ�ȡ11.7g�Ȼ��ƣ�����ȷ��
B����Ͳ����С�̶���0.1mL�����Բ�������Ͳ��ȡ5.26mL���ᣬ�ʴ���
C���㷺pH��ֽ�IJ���ֵ��������������pH��ֽ���ܲⶨ��Һ��pHΪ3.5���ʴ���
D���ζ��ܵĸ�����0.01mL������ȷ��
��ѡAD��
��2��������ˮ��ʪ��ֽ�ᵼ����ҺŨ�Ƚ��ͣ������Һ��ǿ��ǿ���Σ���Һ��pH���䣬������ᡢ����������ӵ�����Һ����Һ��pH��ı䣬������Һ��pH��һ���ı䣻��ˮϡ�ʹٽ�������ʵ��룬��ͬŨ�ȵ�һԪǿ����ϡ����ͬ�ı�����������������Ũ�ȴ���ǿ�ᣬ��������pH�仯С��ǿ�ᣬ������ǿ�ᡢ���������ᣬ�����ϴ�������ᣬ
�ʴ�Ϊ����һ�������
��1��NaOH���и�ʴ�ԣ�Ӧ�÷���С�ձ��г�������ѡA��
��2���ζ�ʱ����0.2000mol?L-1���������ζ�������Һ��ǡ���к�ʱ��Һ��pH=7��ʯ��ı�ɫ��Χ��5-8��ǡ���к�ʱʯ�ﲻ��ɫ��Ӧ��ѡ�ü��Ȼ��̪��ָʾ��������ѡȡʯ���ѡB��
��3���ζ��յ��жϷ����ǣ����������һ�α�����Һʱ����Һ�պ��ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ɫ���ʴ�Ϊ�����������һ�α�����Һʱ����Һ�պ��ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ɫ��
��4���ζ�������ʹ�����ƽ�����=
mL=20.00mL��
�������Ǽ��2�����������ʵ���Ũ�������2��������NaOH��ҺŨ��Ϊ0.4000mol/L��
�ʴ�Ϊ��0.4000��
��5��������ʱ����Һ���ƫС��������ʵ���ƫС��������Һ������䣬���²ⶨ��ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��������ƿ�ô���Һ��ϴ���ᵼ������������ƫ��������ʵ���ƫ����Һ������ǰ�ԭ��������㣬���Բⶨ��ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
B����Ͳ����С�̶���0.1mL�����Բ�������Ͳ��ȡ5.26mL���ᣬ�ʴ���
C���㷺pH��ֽ�IJ���ֵ��������������pH��ֽ���ܲⶨ��Һ��pHΪ3.5���ʴ���
D���ζ��ܵĸ�����0.01mL������ȷ��
��ѡAD��
��2��������ˮ��ʪ��ֽ�ᵼ����ҺŨ�Ƚ��ͣ������Һ��ǿ��ǿ���Σ���Һ��pH���䣬������ᡢ����������ӵ�����Һ����Һ��pH��ı䣬������Һ��pH��һ���ı䣻��ˮϡ�ʹٽ�������ʵ��룬��ͬŨ�ȵ�һԪǿ����ϡ����ͬ�ı�����������������Ũ�ȴ���ǿ�ᣬ��������pH�仯С��ǿ�ᣬ������ǿ�ᡢ���������ᣬ�����ϴ�������ᣬ
�ʴ�Ϊ����һ�������
��1��NaOH���и�ʴ�ԣ�Ӧ�÷���С�ձ��г�������ѡA��
��2���ζ�ʱ����0.2000mol?L-1���������ζ�������Һ��ǡ���к�ʱ��Һ��pH=7��ʯ��ı�ɫ��Χ��5-8��ǡ���к�ʱʯ�ﲻ��ɫ��Ӧ��ѡ�ü��Ȼ��̪��ָʾ��������ѡȡʯ���ѡB��
��3���ζ��յ��жϷ����ǣ����������һ�α�����Һʱ����Һ�պ��ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ɫ���ʴ�Ϊ�����������һ�α�����Һʱ����Һ�պ��ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ɫ��
��4���ζ�������ʹ�����ƽ�����=
| [(20.50-0.40)+(24.00-4.20)+(21.50-0.40)] |
| 3 |
�������Ǽ��2�����������ʵ���Ũ�������2��������NaOH��ҺŨ��Ϊ0.4000mol/L��
�ʴ�Ϊ��0.4000��
��5��������ʱ����Һ���ƫС��������ʵ���ƫС��������Һ������䣬���²ⶨ��ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��������ƿ�ô���Һ��ϴ���ᵼ������������ƫ��������ʵ���ƫ����Һ������ǰ�ԭ��������㣬���Բⶨ��ҺŨ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
���������⿼��̽�����ʺ����ⶨ������к͵ζ������ؿ���ѧ������ʵ�������������������ȷʵ��ԭ���ǽⱾ��ؼ����ѵ������ָʾ����ѡȡ����������ע��ζ��̶ܿȴ�Сλ�ã�Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
�ж����и������Ĺ�ϵΪͬλ�ص��ǣ�������
| A��H2��D2��T2 |
| B��14C��14N |
| C��35Cl��37 Cl- |
| D��16O��17O��18O |
�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������ý��ƫ�ͣ����������ԭ����������������еģ�������
| A���ζ������У���ƿ������Һ���� |
| B����ƿ������ˮϴ����δ�����T���еζ� |
| C����ʽ�ζ���δ�ñ�������ϴ |
| D���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ���ֹʱ������ʧ |
��ͼ����0.1000mol/L��NaOH��Һ�ζ�20.00mLδ֪Ũ�����ᣨ��̪��ָʾ�����ĵζ����ߣ�����˵����ȷ���ǣ�������
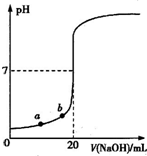

| A��ˮ�������������Ũ�ȣ�a��b |
| B����������ʵ���Ũ��Ϊ0.0100mol?L-1 |
| C��ָʾ����ɫʱ��˵��������NaOHǡ����ȫ��Ӧ |
| D�����μ�NaOH��Һ10.00mLʱ���û��Һ��pH=1+lg3 |
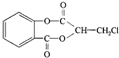 ����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����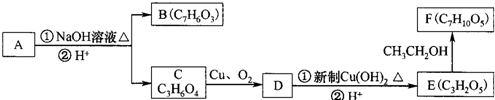
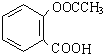 ��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹����
��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹���� �����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
�����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH 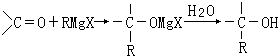
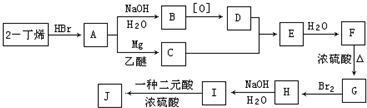

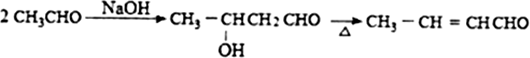
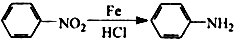 �������а����ױ�������
�������а����ױ�������