��Ŀ����
5��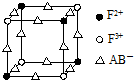 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ�
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ���1����̬��F3+��������Ų�ʽ��1s22s22p63s23p63d5��
��2��B����̬�⻯����ˮ�е��ܽ��Զ����A��C����̬�⻯�ԭ����NH3��H2O���Ӽ���������
��3��������FD3����ɫ���塢�׳��⡢100������ʱ���������ľ��������Ƿ��Ӿ��壻������ECAB�е���������AC2��Ϊ�ȵ����壬�������ӵĵ���ʽ��
 ��
����4��������EF[F��AB��6]��һ����ɫ���壬��ͼ��ʾ�侧����$\frac{1}{8}$��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ4��
��5��������ECAB��������Ũ�����ϼ��ȣ�������������֣�����һ�ֳʺ���ɫ�������־�Ϊ��ɫ�������������嶼�ܲ������ѭ��������Ӿ�Ϊ�Ǽ��Է��ӣ�д���÷�Ӧ�����ӷ���ʽ2SCN-+20 H++22 NO3-=2SO42-+2CO2��+N2��+22NO2��+10H2O��
���� A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2������A��CԪ�أ�C��SԪ�أ�����ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��E��KԪ�أ�D��ԭ����������S��KԪ��֮�䣬��DΪClԪ�أ�B�ĵ縺�Դ���C����B��ԭ������С��C�����ڲ�ͬ���壬����B��NԪ�أ�Fλ�ڵ������ڣ���̬ԭ������4��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3d64s2����F��FeԪ�أ��ݴ˽��
��1�����ݺ�������Ų�������д��̬��Fe3+��������Ų�ʽ��
��2����ˮ֮���γ�������������ʵ��ܽ��ԣ�
��3��������FeCl3����ɫ���塢�׳��⡢100������ʱ�������۷е�ͣ����ڷ��Ӿ��壻KSCN�е���������CS2��Ϊ�ȵ����壬�ṹ���ƣ�
��4�����ݾ�̯�����㣬��$\frac{1}{8}$�����к���Fe2+��Fe3+��CN-��Ŀ�����ݻ��ϼ۴�����Ϊ0����$\frac{1}{8}$�����к���K+��Ŀ����������һ��������K+�ĸ�����
��5��������KSCN��������Ũ�����ϼ��ȣ�������������֣�����һ�ֳʺ���ɫΪNO2�������־�Ϊ��ɫ�������������嶼�ܲ������ѭ��������Ӿ�Ϊ�Ǽ��Է��ӣ��ֱ�ΪCO2��N2�������������Σ�
��� �⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2������A��CԪ�أ�C��SԪ�أ�����ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��E��KԪ�أ�D��ԭ����������S��KԪ��֮�䣬��DΪClԪ�أ�B�ĵ縺�Դ���C����B��ԭ������С��C�����ڲ�ͬ���壬����B��NԪ�أ�Fλ�ڵ������ڣ���̬ԭ������4��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3d64s2����F��FeԪ�أ�
��1����̬��Fe3+��������Ų�ʽ�ǣ�1s22s22p63s23p63d5���ʴ�Ϊ��1s22s22p63s23p63d5��
��2��B����̬�⻯��ΪNH3��A��C����̬�⻯��ֱ�ΪCH4��H2S��NH3��H2O���Ӽ�����������CH4��H2S������ˮ�����γ��������NH3��ˮ�е��ܽ��Զ����CH4��H2S��
�ʴ�Ϊ��NH3��H2O���Ӽ���������
��3��������FeCl3����ɫ���塢�׳��⡢100������ʱ�������۷е�ͣ����ڷ��Ӿ��壬������KSCN�е���������CS2��Ϊ�ȵ����壬�������ӵĵ���ʽ�� ��
��
�ʴ�Ϊ�����Ӿ��壻 ��
��
��4����$\frac{1}{8}$�����к���Fe2+��4��$\frac{1}{8}$=$\frac{1}{2}$����Fe3+��4��$\frac{1}{8}$=$\frac{1}{2}$����CN-��12��$\frac{1}{4}$=3�����ݻ��ϼ۴�����Ϊ0��ԭ��$\frac{1}{8}$�����к���K+��3-��$\frac{1}{2}$��2+$\frac{1}{2}$��3��=$\frac{1}{2}$����һ��������K+�ĸ���Ϊ$\frac{1}{2}$��8=4��
�ʴ�Ϊ��4��
��5��������KSCN��������Ũ�����ϼ��ȣ�������������֣�����һ�ֳʺ���ɫΪNO2�������־�Ϊ��ɫ�������������嶼�ܲ������ѭ��������Ӿ�Ϊ�Ǽ��Է��ӣ��ֱ�ΪCO2��N2�������������Σ��ʸ÷�Ӧ�����ӷ���ʽ��2SCN-+20 H++22 NO3-=2SO42-+2CO2��+N2��+22NO2��+10H2O��
�ʴ�Ϊ��2SCN-+20 H++22 NO3-=2SO42-+2CO2��+N2��+22NO2��+10H2O��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų���������������������ʡ��ȵ����塢������������ȣ���4����ע�����þ�̯�����м��㣬�Ѷ��еȣ�

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�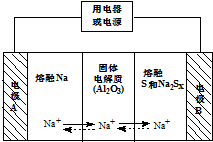 �����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ���ԭ����ͼ��ʾ��Na2SX$?_{�ŵ�}^{���}$2Na+xS ��3��x��5��
�����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ���ԭ����ͼ��ʾ��Na2SX$?_{�ŵ�}^{���}$2Na+xS ��3��x��5��| ���� | Na | S | Al2O3 |
| �۵�/�� | 97.8 | 115 | 2050 |
| �е�/�� | 892 | 444.6 | 2980 |
A��100������ B��100�桫300��C��300�桫350��D��350�桫2050��
��2�����������أ�����˵����ȷ����ACD������ĸ��ţ���
A���ŵ�ʱ���缫AΪ����
B���ŵ�ʱ��Na+���ƶ�����Ϊ��B��A
C���ŵ�ʱ��������ӦʽΪ 2Na-2e-=2Na+
D�����ʱ�缫B�ĵ缫��ӦʽΪSX2--2e-=xS
��3��25��ʱ��������������Ϊ��Դ���200mL 0.2mol/L NaCl��Һ������Һ��pH��Ϊl3ʱ����·��ͨ���ĵ��ӵ����ʵ���Ϊ0.02mol�������ķ�Ӧ���������Ϊ0.92g����������ǰ�����ķ�Ӧ�����������Һ�������仯��
 ���Ļ�������ũҵ��������ҵ�����칤ҵ�������й㷺����;��
���Ļ�������ũҵ��������ҵ�����칤ҵ�������й㷺����;����1�����칤ҵ�г���N2H4������ȼ�ϣ�N2O4������������֪N2��g��+O2��g��=2NO��g����H=+180.7kJ•mol-1
2NO��g��+O2��g��=2NO2��g����H=-113.0kJ•mol-1
N2H4��g��+O2��g��=N2��g��+2H2O��g����H=-534.0kJ•mol-1
2NO2��g��?N2O4��g����H=-52.7kJ•mol-1
N2H4��g����N2O4��g����Ӧ����һ����̬��10e-���ӣ�����һ�ּ��ȶ��ĵ��ʣ�д����Ӧ���Ȼ�ѧ����ʽ��2N2H4��g��+N2O4��g��?3N2��g��+4H2O��g����H=-1083.0kJ•mol-1
��2����ҵ�ϳɰ����������ķ�չ�����ش���ʵ�����г���N2��H2��һ�������½��кϳɰ�������о���T��ʱ�����ݻ�Ϊ3L���ܱ������У�Ͷ��4mol N2��9mol H2��10min�ﵽ��ѧƽ��״̬��ƽ��ʱNH3�����ʵ���Ϊ2mol����0��10min��H2��ƽ������v��H2��=0.1mol/��L��min����ƽ��ʱN2��ת���ʦ���N2��=25%��������������Ũ�ȣ��÷�Ӧ��ƽ�ⳣ�������䣨���������С�����䡱����
��3����ˮ��һ�ֳ��õij��������кͼ���
����֪25��ʱ���������ܵ���ʵ��ܶȻ����±���ʾ��
| �������� | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| Ksp | 2.2��10-20 | 4.0��10-38 | 8.0��10-16 | 1.8��10-11 |
��25��ʱ����amol/L�İ�ˮ��b mol/L����������ϣ���Ӧ����Һǡ�������ԣ���a��b�� �����������������=��������a��b��ʾNH3•H2O�ĵ���ƽ�ⳣ��Kb=$\frac{b��1{0}^{-7}}{a-b}$��
��4����NH4��2CO3��һ�ֲ�̼�����䲶CO2��ԭ��Ϊ��
��NH4��2CO3��aq��+H2O��l��+CO2��g�� 2NH4HCO3 ��aq����H
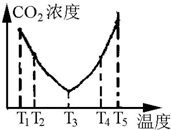
Ϊ�о��¶ȶԲ�̼Ч�ʵ�Ӱ�죬�ڲ�ͬ�¶������£���һ�����ģ�NH4��2CO3��Һ�����ܱ������У�������һ������CO2����tʱ�̣����������CO2�����Ũ�ȣ����ϵ��ͼ��
�ٲ�CO2��Ӧ�ġ�H��0�����������=����������
����T4��T5����¶����䣬������CO2����Ũ�ȱ仯���Ƶ�ԭ���ǣ�T4��T5��Ӧ��ƽ�⣬����ӦΪ���ȷ�Ӧ�������¶ȵ����ߣ�ƽ�������ƶ���CO2������Ч�ʽ��ͣ���NH4HCO3���ַֽ⣩��
| A�� | S2Cl2����������H2O����ԭ�� | |
| B�� | ÿ����l mol SO2ת��4 mol���� | |
| C�� | ���������뻹ԭ��������ʵ���֮��Ϊl��3 | |
| D�� | ÿ����48g������2mol������ |
| A | B | C | D | |
| ��Ⱦ | �Ͼ��ȹ������� | úȼ�� | ��Hg2+�Ĺ�ҵ��ˮ | �������� |
| ���� | ������Ϊ���� | ú�м�������ʯ��ʯ | ����Na2S��Һ | ���շ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ҹ�����ͳ��ʳ������Ϊ�������ۡ�֬�������������� | |
| B�� | �����ڵĵ����ʲ��Ϸֽ⣬��������ˮ�Ͷ�����̼�ų����� | |
| C�� | ������ˮ��ˮ���ڱ������γ�һ��ˮ����������ʳ�׳�ȥ | |
| D�� | ������ˮʱ�����˻�ѧ�������仯������ɱ�������������� |
| A�� | CH3CH��CH2CH3��2��ϵͳ����Ϊ3-�����飬��CH3CH2CH��CH3��CH2CH3��Ϊͬϵ�� | |
| B�� | ��ϩ������ϩ�;�����ϩ���������ӳɷ�Ӧ��ʹ��ˮ��ɫ | |
| C�� | �øʰ��ᣨ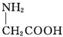 ���ͱ����ᣨ ���ͱ����ᣨ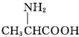 ���Ļ������һ�������¿��γ�������״���� ���Ļ������һ�������¿��γ�������״���� | |
| D�� | �ױ���C7H8�����ͣ�C3H8O3��������������һ��ʱ����$\frac{n��{C}_{7}{H}_{8}��}{n��{C}_{3}{H}_{8}{O}_{3}��}$��ֵ��������������ˮ���������� |
| A�� | 11.2g | B�� | 22.4g | C�� | 5.6g | D�� | 56g |