��Ŀ����
17��������ܻ����ڱ�����������������ڱ�������������������Ũ���Ṳ���·�Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽ��װ��ͼ������װ��ʡ�ԣ���ͼ1��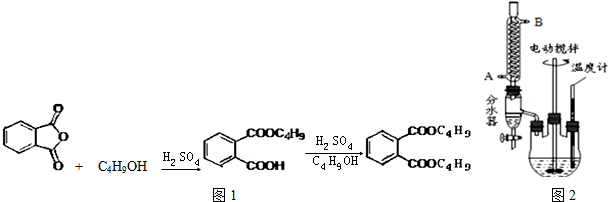
��֪���������е�118�棬���ڱ����������������ɫ�������з�����ζ����״Һ�壬�е�340�棬���������£��¶ȳ���180��ʱ�����ֽ⣮���ڱ������������������Ʊ��ڱ������������ʵ������������£�
����������ƿ�ڼ���30g��0.2mol���ڱ�����������22g��0.3mol���������Լ�����Ũ���ᣮ
�ڽ��裬������105�棬�������跴Ӧ2Сʱ����������Ӧ������
����ȴ�����£�����Ӧ����ﵹ����ͨ�����������еIJ���X���õ��ֲ�Ʒ��
�ֲܴ�Ʒ����ˮ����þ�����������ȡ��Һ����������Բ����ƿ����ѹ�����������õ���Ʒ20.85g��
��ش��������⣺
��1��������в��ϴӷ�ˮ���²����������ˮ��Ŀ���������ڷ�Ӧ�������ڱ�������������ķ����ƶ�����߲��ʣ��жϷ�Ӧ�ѽ����ķ����Ƿ�ˮ���е�ˮλ�߶Ȼ������ֲ���ʱ�������������в�����Һ����£���
��2������ʵ��������ɵĸ�����Ľṹ��ʽΪCH2=CHCH2CH3��CH3CH2CH2CH2OCH2CH2CH2CH3�ȣ���һ�ּ��ɣ�
��3������X�У�Ӧ����5%Na2CO3��Һϴ�Ӵֲ�Ʒ��������ҺŨ�Ȳ��˹��ߣ�������ʹ���������ƣ���ʹ������������Һ���Բ�����ʲôӰ�죿���û�ѧ����ʽ��ʾ��
 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$ +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH����4������X�У����������IJ����б���ʹ�õ���Ҫ���������з�Һ©�����ձ���
��5���ֲ�Ʒ�ᴿ�����в��ü�ѹ�����Ŀ�����ڱ�������������е�ϸߣ���������������ֽ⣬��ѹ��ʹ��е㽵�ͣ�
��6����ʵ���У��ڱ��������������ʽ����278���IJ���Ϊ50%��
���� ��1��ˮ����������ϵķ��������ʹƽ�����������ƶ���������߷�Ӧ���ת���ʣ���Ӧ����ʱ����ˮ���е�ˮλ�߶Ȳ��䣬�������в�����Һ����£�
��2�����������ܷ�����ȥ��Ӧ��Ҳ���Է������Ӽ���ˮ��Ӧ�����ѵȣ�
��3����ʹ������������Һ���ᷢ���ڱ�������������ڼ��������µ�ˮ�ⷴӦ���� ����������
����������
��4������X�ǽ��������ܵ�Һ����з��룬Ӧ��ȡ��Һ������
��5���ڱ�������������ķе�340�棬�¶ȳ���180��ʱ�����ֽ⣬Ӧ��ѹ����ʹ��е㽵�ͣ���ֹ�ֽ⣻
��6���������������㣬��������������ȫת�����Դ˼����ڱ�����������������۲���������=��ʵ�ʲ��������۲�������100%��
��� �⣺��1��ˮ����������ϵķ��������ʹƽ�����������ƶ���������߷�Ӧ���ת���ʣ���ˮ���е�ˮλ�߶Ȼ������ֲ���ʱ�������������в�����Һ����£���˵����Ӧ������
�ʴ�Ϊ�������ڷ�Ӧ�������ڱ�������������ķ����ƶ�����߲��ʣ���ˮ���е�ˮλ�߶Ȼ������ֲ���ʱ�������������в�����Һ����£���
��2�����������ܷ�����ȥ��Ӧ��Ҳ���Է������Ӽ���ˮ��Ӧ�����ѵȣ�ʵ���и�����Ľṹ��ʽΪ��CH2=CHCH2CH3 ��CH3CH2CH2CH2OCH2CH2CH2CH3�ȣ�
�ʴ�Ϊ��CH2=CHCH2CH3��CH3CH2CH2CH2OCH2CH2CH2CH3�ȣ�
��3����ʹ������������Һ���ᷢ���ڱ�������������ڼ��������µ�ˮ�ⷴӦ���� ������������Ӧ����ʽΪ��
������������Ӧ����ʽΪ�� +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$ +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH��
�ʴ�Ϊ�� +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$ +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH��
��4������X�ǽ��������ܵ�Һ����з��룬Ӧ��ȡ��Һ�����������б���ʹ�õ���Ҫ���������У���Һ©�����ձ���
�ʴ�Ϊ����Һ©�����ձ���
��5���ڱ�������������е�ϸߣ���������������ֽ⣬��ѹ��ʹ��е㽵�ͣ���ֹ�ֽ⣬
�ʴ�Ϊ���ڱ�������������е�ϸߣ���������������ֽ⣬��ѹ��ʹ��е㽵�ͣ�
��6���������������㣬������������ȫת�������ڱ�����������������۲���Ϊ��$\frac{0.3mol}{2}$��278g/mol=41.7g���������Ϊ$\frac{20.85g}{41.7g}$��100%=50%��
�ʴ�Ϊ��50%��
���� ���⿼���Ʊ���������ƣ���Ŀ�Ѷ��еȣ��漰���ʵķ����ᴿ���Բ�����ԭ���ķ������ۡ����ʼ����֪ʶ������ʵ�������Ҫ���ʵ��ԭ���ǽ���Ĺؼ�������������ѧ���ķ�����������ѧʵ��������

| A�� | 360 kJ/mol | B�� | 263 kJ/mol | C�� | 1 173 kJ/mol | D�� | 391 kJ/mol |
 �������ʵ���Ũ�ȵ�CuSO4��Һ��NaCl��Һ�������Ϻ���ʯī�缫���е�⣬�������ҺpH��ʱ��t�仯��������ͼ��������˵����ȷ���ǣ�������
�������ʵ���Ũ�ȵ�CuSO4��Һ��NaCl��Һ�������Ϻ���ʯī�缫���е�⣬�������ҺpH��ʱ��t�仯��������ͼ��������˵����ȷ���ǣ�������| A�� | ��������һ����Cl2����������һ����Cu | |
| B�� | BC�α�ʾ����������H+�ŵ������H2 | |
| C�� | CD�α�ʾ���ˮ | |
| D�� | CD�α�ʾ������OH-�ŵ��ƻ���ˮ�ĵ���ƽ�⣬������H+ |
| A�� | ��ȫȼ�����ɶ�����̼��ˮ�Ļ����ﲻһ������ | |
| B�� | ��ͬ��������������춡��ֱ���ȫȼ�գ���������� | |
| C�� | ��ͬ���ʵ�����ϩ���Ҵ��ֱ���ȫȼ�գ���������� | |
| D�� | ��ͬ�������������Ȳ�ֱ���ȫȼ�գ���������� |

��1������������ʵ���Ũ����________mol/L��
��2��ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ490 mL 4.6 mol/L��ϡ���ᣬ����Ҫȡ________mL�ĸ����ᡣ
��3������ʱ������IJ�����������Ͳ���ձ����������ͽ�ͷ�ι�֮�⣬����Ҫ �����������ƣ���
��4��������Һ�����£�δ��˳�����У���a���ܽ⣬b��ҡ�ȣ�c��ϴ�ӣ�d����ȴ��e��������f������Һ��������ƿ��g.���ݵȲ����� ����ҡ�ȵ�ǰһ�������� ������д��ĸ��
��5�����������ƹ���ʾ��ͼ�У��д�����ǣ���д��ţ� ��

��6��������4.6 mol/L��ϡ����Ĺ����У��������������������Һ���ʵ���Ũ��ƫ�ߵ���_________
A��δ����ȴ���Ƚ���Һע������ƿ�� | B������ƿϴ�Ӻ�δ�����ﴦ�� |
C������ʱ���ӹ۲�Һ�� | D��δϴ���ձ��Ͳ����� |
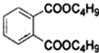
 С��ͬѧ�������ͼ��ʾװ��̽��ͭ��Ũ����ķ�Ӧ���ȹرջ���a����������ƿ�в��������ݲ���ʱ����Ӧֹͣ����ʱ��ƿ��ͭƬ����ʣ�࣮�����ٴ���a���������е���������������ƿ��ͭƬ�������٣�
С��ͬѧ�������ͼ��ʾװ��̽��ͭ��Ũ����ķ�Ӧ���ȹرջ���a����������ƿ�в��������ݲ���ʱ����Ӧֹͣ����ʱ��ƿ��ͭƬ����ʣ�࣮�����ٴ���a���������е���������������ƿ��ͭƬ�������٣�

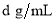 �������Һ�����ʵ���Ũ��Ϊ mol/L
�������Һ�����ʵ���Ũ��Ϊ mol/L B��
B�� C��
C�� D��
D��