��Ŀ����
�����״����飩�й����ʵ���Ũ�ȵļ���
��1����4gNaOH��������ˮ���250mL��Һ������Һ��NaOH�����ʵ���Ũ��Ϊ_________mol/L��ȡ��10mL����Һ�����к���NaOH_________g����ȡ������Һ��ˮϡ�͵�100mL��ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ_________mol/L��

��2����ͼʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��ݴ˼��㣺��Ũ������HCl�����ʵ���Ũ��Ϊ__________mol/L��������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol/L��ϡ���ᡣ��Ҫ��ȡ___________mL����Ũ����������ơ�
��3��100mL0.3mol/LNa2SO4��Һ��50mL0.2mol/LAl2(SO4)3��Һ��Ϻ���Һ��SO42�������ʵ���Ũ��Ϊ__________mol/L
��4����״���£���V L A���壨Ħ������ΪM g/mol������0.1Lˮ(�ܶ�1 g/cm3)�У�������Һ���ܶ�Ϊ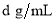 �������Һ�����ʵ���Ũ��Ϊ mol/L
�������Һ�����ʵ���Ũ��Ϊ mol/L
A�� B��
B�� C��
C�� D��
D��
ij�жԴ������м�⣬������Ҫ��Ⱦ��Ϊ�����������PM2.5������Ҫ��ԴΪȼú��������β���ȡ���PM2.5����������ˮ�����Ƴ���Һ������Һ������ˮ���������ӵ�Ũ�����±���
���� | K+ | Na+ | NH4+ | H + | SO42�� | NO3�� | Cl�� |
Ũ��/mol��L��1 | 4��10��6 | 6��10��6[ | 2��10��5 | x | 4��10��5 | 3��10��5 | 2��10��5 |
���ݱ��������ж�H+ ��Ũ��Ϊ mol��L��1
A��1��10��5 mol��L��1 B��3��10��5 mol��L��1
C��6��10��5 mol��L��1 D��1��10��4mol��L��1
 ij�о�С���ñ�NaOH��Һ�ζ��״ף��ⶨʳ�ð״��д���ĺ���������˵����ȷ���ǣ�������
ij�о�С���ñ�NaOH��Һ�ζ��״ף��ⶨʳ�ð״��д���ĺ���������˵����ȷ���ǣ�������| A�� | ����ͼ����ȡһ������Ĵ���״�����ƿ�� | |
| B�� | �۲��ʽ�ζ��ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ������ᵼ��ʳ�ð״��д���ĺ���ƫС | |
| C�� | ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�������ᵼ��ʳ�ð״��д���ĺ���ƫ�� | |
| D�� | �ζ�ʱ�۾�Ҫע���ŵζ�����NaOH��Һ��Һ��仯����ֹ�ζ����� |


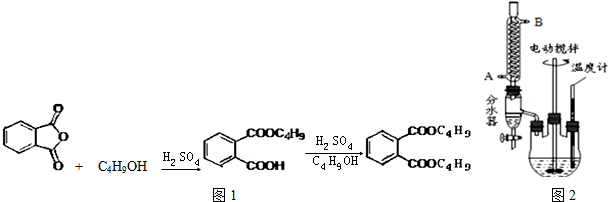
 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$ +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH�� 10��9m~10��7m��Χ�ڣ���ԭ�ӡ����ӽ��в��ݵ����׳����Ӽ���������ʵ�����벻���ı仯������ͭ��һ���������ͻ����ȼ�գ�����������ը������˵����ȷ����
10��9m~10��7m��Χ�ڣ���ԭ�ӡ����ӽ��в��ݵ����׳����Ӽ���������ʵ�����벻���ı仯������ͭ��һ���������ͻ����ȼ�գ�����������ը������˵����ȷ���� )
) 2C(g)��2 s�ڵķ�Ӧ���ʣ�v(A2)=0��5 mol��L-1��s-1��v(B2)=1��5 mol��L-1��s-1��v(C)=1 mol��L-1��s-1����x��y��ֵ�ֱ�Ϊ
2C(g)��2 s�ڵķ�Ӧ���ʣ�v(A2)=0��5 mol��L-1��s-1��v(B2)=1��5 mol��L-1��s-1��v(C)=1 mol��L-1��s-1����x��y��ֵ�ֱ�Ϊ 2AB3(g)��Ӧ��˵,���»�ѧ��Ӧ���ʵı�ʾ��,��ѧ��Ӧ����������
2AB3(g)��Ӧ��˵,���»�ѧ��Ӧ���ʵı�ʾ��,��ѧ��Ӧ����������