��Ŀ����
2��NH4Al��SO4��2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺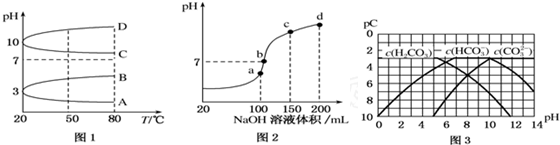
��1����ͬ�����£�������ʵ���Ũ�ȵ�NH4Al��SO4��2��c��NH${\;}_{4}^{+}$��С�ڣ�����ڡ������ڡ���С�ڡ���0.1mol•L-1 NH4HSO4��c��NH${\;}_{4}^{+}$����
��2����ͼ1��0.1mol•L-1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1mol•L-1NH4Al��SO4��2��pH���¶ȱ仯��������A����д��ĸ����
������ʱ��0.1mol•L-1NH4Al��SO4��2��2c��SO${\;}_{4}^{2-}$��-c��NH${\;}_{4}^{+}$��-3c��Al3+��=10-3mol•L-1������ֵ����
��3������ʱ����100mL0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������a����b�㣬��Һ�и�����Ũ���ɴ�С������˳����c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ��1�������ӡ�����������笠�����ˮ�⣬�����������Ƴ̶�С�������ӣ�
��2����NH4Al��SO4��2Ϊǿ�������Σ�����Һ�����ԣ������¶ȴٽ�ˮ�⣻
�ڸ�����Һ�еĵ���غ���㣻
��3��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��b��c��d������Һ������NH3•H2O����NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3•H2O����ˮ�ĵ��룮b����Һ�����ԣ�
��� �⣺��1��NH4Al��SO4��2��NH4HSO4�е�NH4+������ˮ�⣬����NH4Al��SO4��2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣬��ΪHSO4-�������ɵ�H+Ũ�ȱ�Al3+ˮ�����ɵ�H+Ũ�ȴ�����NH4HSO4��NH4+ˮ��̶ȱ�NH4Al��SO4��2�е�С��
�ʴ�Ϊ��С�ڣ�
��2����NH4Al��SO4��2Ϊǿ�������Σ�����Һ�����ԣ������¶ȴٽ�ˮ�⣬������Һ������ǿ����Һ��pH��С��������A���ϣ�
�ʴ�Ϊ��A��
�ڸ��ݵ���غ�ɵã�2c��SO42-��-c��NH4+��-3c��Al3+��=c��H+��-c��OH-��=10-3 mol•L-1[c��OH-��̫С���ɺ���]��
�ʴ�Ϊ��10-3��
��3��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��b��c��d������Һ������NH3•H2O����NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3•H2O����ˮ�ĵ��룮b����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�a��ʱc��Na+��=c��SO42-����b��ʱc��Na+����c��SO42-��������NԪ����SԪ�صĹ�ϵ�����Եó�c��SO42-����c��NH4+������c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
�ʴ�Ϊ��a��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ���⿼��������Ũ�ȴ�С�Ƚϡ�����ˮ���֪ʶ�㣬��Ŀ�Ѷ��еȣ�����Ũ�ȴ�С�Ƚϳ���������ˮ�⡢������ʵĵ������Ͽ��飬ȷ������Ũ�ȴ�СʱҪ��ϵ���غ㡢�����غ�����������ѵ��ǣ�3���⣬֪��ͼ���и���������ʼ��ɽ��

| A�� | H+��Na+��HCO${\;}_{3}^{-}$��Cl- | B�� | Mg2+��NO${\;}_{3}^{-}$��Na+��OH- | ||
| C�� | Ca2+��Cl-��CO${\;}_{3}^{2-}$��OH- | D�� | Na+��Cl-��K+��NO${\;}_{3}^{-}$ |
| A�� | ��16 g��ԭ�ӵĶ������辧���к��еĦļ���ĿΪ2NA | |
| B�� | 23.4 g NaCl�����к���0.1NA����ͼ��ʾ�Ľṹ��Ԫ | |
| C�� | ���³�ѹ�£�5 g D2O���е�������������������������Ϊ2.5NA | |
| D�� | 2 mol SO2��1 mol O2��һ�������·�Ӧ���û�����������С��2NA |
| A�� | ����������Һ����̼���ƺ��Ȼ��� | |
| B�� | �����ᱵ��Һ����̼���ƺ������� | |
| C�� | ���Ȼ�����Һ����̼���ƺ�̼������ | |
| D�� | �����������������ƺ��Ȼ��� |
| A�� | 1��10-11mol/L | B�� | 1��10-3mol/L | C�� | 1��10-7mol/L | D�� | 0.1mol/L |
| A�� | ��ͬ�¶�ʱ��Na2CO3 ���ܽ��С��NaHCO3 ���ܽ�� | |
| B�� | ��ȥ̼�����ƹ����л��е�����̼���ƿ��Բ��ü��ȵķ��� | |
| C�� | ��ͬŨ��ϡ���ᷴӦ��NaHCO3�ų����ݵ����ʸ��� | |
| D�� | �����������Һ���ٷֱ�μ�Ca��OH��2��Һ���ް�ɫ�������ɵ���NaHCO3 |
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |