��Ŀ����
15�� ͼ������ǻ�ѧ�о���һ����Ҫ���ֶΣ�ijͬѧ����ij��ɫ����Һ����֪���п��ܺ���Fe3+��Mg2+��Cu2+��Al3+��NH4+�����뵭��ɫ��ĩ���������ƣ������ȣ�������������������ʵ����뵭��ɫ��ĩ�����ʵ�����ϵ��ͼ��ʾ���������������ȫ���ݳ�������ش�
ͼ������ǻ�ѧ�о���һ����Ҫ���ֶΣ�ijͬѧ����ij��ɫ����Һ����֪���п��ܺ���Fe3+��Mg2+��Cu2+��Al3+��NH4+�����뵭��ɫ��ĩ���������ƣ������ȣ�������������������ʵ����뵭��ɫ��ĩ�����ʵ�����ϵ��ͼ��ʾ���������������ȫ���ݳ�������ش���1������ͼ����ʾ���Ʋ���Һ�п϶���Mg2+��Al3+��NH4+���ӣ���д��ѧʽ��
��2����д�������й����������ʵ�����0.3��0.35molʱ���ֵ����߳����»������������������ӷ�Ӧ����ʽ���ù���2Na2O2+2H2O=4Na++4OH-+O2����Al��OH��3+OH-�TAlO2-+2H2O��
��3����Һ�����ӵ����ʵ���֮��Ϊ1��1��1��
���� ��ɫ��Һ�в�������ɫ��Fe3+��Cu2+���ӣ�����ͼ���֪������������ƺ����ɳ�������������0.3mol�������ƺ������ʼ�ܽ⣬֮�����������������䣬˵��ԭ��Һ��һ������Mg2+��Al3+����0.3-0.35mol��һ���ڣ����ܽ�������������0.1mol�������Al3+��Al��OH��3��[Al��OH��4]-�����������ӵ����ʵ�����0.1mol����0��0.3mol����������һ�Σ����ɳ����������������þ�����������Ļ������Ժ���������þҲ��0.1mol������Mg2+��Mg��OH��2������þ���ӵ����ʵ�����0.1mol���������������������0.3mol��������ʱ�����ݷ�Ӧ2Na2O2+2H2O=4NaOH+O2����֪�����ϻ����0.15mol������������ʱ�������干0.25mol�����Ի�����0.1mol�İ������ݴ��жϼ��ɣ�
��� �⣺��1����ɫ��Һ�в�������ɫ��Fe3+��Cu2+���ӣ�����ͼ���֪������������ƺ����ɳ�������������0.3mol�������ƺ������ʼ�ܽ⣬֮�����������������䣬˵��ԭ��Һ��һ������Mg2+��Al3+���������������������0.3mol��������ʱ�������ϻ����0.15mol����������ʱ�������干0.25mol�����Ի�����0.1mol�İ�����˵��һ������NH4+��
���ݷ�����֪��ԭ��Һ��һ�����е�����Ϊ��Mg2+��Al3+��NH4+��
�ʴ�Ϊ��Mg2+��Al3+��NH4+��
��2�������������ʵ�����0.3��0.35molʱ��������þ���������Ʋ���Ӧ�����������������Ժ��������Ʒ�����Ӧ��������Ӧ�����ӷ���ʽ�У�2Na2O2+2H2O=4Na++4OH-+O2����Al��OH��3+OH-�TAlO2-+2H2O��
�ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH-+O2����Al��OH��3+OH-�TAlO2-+2H2O��
��3���������������ˮ��Ӧ��2Na2O2+2H2O=4NaOH+O2������0.3-0.35mol��һ���ڣ����ܽ�������������0.1mol�������Al3+��Al��OH��3��[Al��OH��4]-�����������ӵ����ʵ�����0.1mol����0��0.3mol����������һ�Σ����ɳ����������������þ�����������Ļ������Ժ���������þҲ��0.1mol������Mg2+��Mg��OH��2������þ���ӵ����ʵ�����0.1mol���������������������0.3mol��������ʱ�������ϻ����0.15mol����������ʱ�������干0.25mol�����Ի�����0.1mol�İ���������NH4+��NH3����笠����ӵ����ʵ���Ϊ��0.1mol��
���ݷ�����֪��Һ�д��ڣ�0.1molMg2+��0.1molNH4+��0.1molAl3+���������ӵ����ʵ���֮��=0.1mol��0.1mol��0.1mol=1��1��1��
�ʴ�Ϊ��1��1��1��
���� ���⿼�������ӷ���ʽ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷͼ�����߶�Ӧ��Ӧԭ��Ϊ���ؼ���ע���������ճ���Ԫ�ؼ��仯�������ʣ�������ؿ���ѧ���ķ�����������������ѧ����������

| A�� | x=0.5��a=8 | B�� | x=0.5��a=10 | C�� | x=1.5��a=8 | D�� | x=1.5��a=10 |
�����ӷ�Ӧ������ʵ�����
�������¶�
����С��Ӧ���������
�ܲ��Ϸ����������
�ݼ���MnO2��
| A�� | ȫ�� | B�� | �٢ڢ� | C�� | �ڢ� | D�� | �� |
| A�� | �����ʵ���Ũ�ȵ�NaCl��AlCl3���Һ��ȫ�������õĻ��Һ��pHֵ���� | |
| B�� | NaCl��Һ���һ��ʱ���Ҫ�ָ������ǰ״̬��Ӧ������������ | |
| C�� | Na2SO4��Һ�ڵ������У�������pHֵ���� | |
| D�� | ���CuSO4��Һ��������ӦʽΪ��2H2O+O2+4e-=4OH- |
| A�� | SO2���������������ǻ�ԭ���� | |
| B�� | CuFeS2������ԭ������Ԫ�ر����� | |
| C�� | ÿ����1mol Cu2S����4 mol������ | |
| D�� | ÿת��1.2 mol���ӣ���0.3 mol������ |
 Ӫ��Ʒ��ҩƷ���DZ�֤���ཡ������ȱ�ٵ����ʣ������ʺ��Ʒ��ǻ�ѧ�о�����Ҫ���ݣ���֪�Ұ�����һ�����������ȱ�ٵİ����ᣬ���Ľṹ��ʽ�ǣ�
Ӫ��Ʒ��ҩƷ���DZ�֤���ཡ������ȱ�ٵ����ʣ������ʺ��Ʒ��ǻ�ѧ�о�����Ҫ���ݣ���֪�Ұ�����һ�����������ȱ�ٵİ����ᣬ���Ľṹ��ʽ�ǣ� ��
��

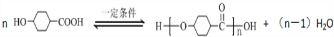 ��
�� ���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ
���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ ��
�� ��
��