��Ŀ����
11����1����һѧ����ʵ���Ҳ�ij��ҺpH��ʵ��ʱ������������ˮ��ʪpH��ֽ��Ȼ���ýྻ����IJ�����պȡ�������м�⣮�����ִ��������һ�����һ����/��һ������/����һ�������ᵼ��ʵ��������
�������˷��ֱ�ⶨc��H+����ȵ�����ʹ�����Һ��pH�����ϴ�������ᣮ
��2������֪Ũ�ȵ� NaOH ��Һ�ⶨij HCl��Һ��Ũ�ȣ��ο���ͼ���ӱ���ѡ����ȷ���
| ��� | ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� |  ѡ�õζ��� |
| A | �� | �� | ʯо | ���ң� |
| B | �� | �� | ��̪ | ���ף� |
| C | �� | �� | ���� | ���ף� |
| D | �� | �� | ��̪ | ���ң� |
A�����Ʊ���Һ�����������л���Na2CO3����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
D���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
E��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ�
���� ��1���ٸ�����Һϡ�ͺ���Һ������Ա仯��pHֵ�Ĺ�ϵ���н����Һ������ʱ����������ֵû����
����ˮ��ʪ�൱��ϡ�ͻ�ٽ�������ʵĵ��룻
��2����֪Ũ�ȵ� NaOH ��Һ�ⶨij HCl��Һ��Ũ�ȣ���ƿ��Ϊ�ᣬ�ζ�����ʢ��NaOH��Һ��ѡ���̪Ϊָʾ����
��3���ñ���NaOH�ζ�δ֪Ũ�ȵ����ᣬѡ�÷�̪Ϊָʾ�������c��=$\frac{cV{\;}_{��}}{V{\;}_{��}}$������
��� �⣺��1����������Һ��ˮϡ�ͺ�pH���䣻������Һϡ�ͺ���Һ���Լ�����pH�������Һϡ�ͺ��Ա�С��pHֵ����С�����Բⶨ�Ľ����һ����������������Һ�䣻
�ʴ�Ϊ����һ����
����ˮ��ʪ�൱��ϡ�ͣ��������PHƫ������ϡ�ͻ�ٽ�������ʵĵ��룬ϡ�����д����������������ӣ�ʹ����Һ��������Ũ�ȱ仯�������С���������PH���ʴ�Ϊ�����
��2����֪Ũ�ȵ� NaOH ��Һ�ⶨij HCl��Һ��Ũ�ȣ���ƿ��Ϊ�ᣬ�ζ�����ʢ��NaOH��Һ��ͼ�еζ���ѡ���ң�ѡ���̪Ϊָʾ�����ʴ�Ϊ��D��
��3��A�����Ʊ���Һ�����������л���Na2CO3���ʣ�������������������Һ���ƫ����c��=$\frac{cV{\;}_{��}}{V{\;}_{��}}$��֪���ⶨ��Ũ��ƫ�ߣ���Aѡ��
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ�������ƫС��V��NaOH��ƫС����c��=$\frac{cV{\;}_{��}}{V{\;}_{��}}$��֪���ⶨ��Ũ��ƫС����B��ѡ��
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ��������ʵ������䣬ʵ����Ӱ�죬��C��ѡ��
D���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ��V��NaOH��ƫ�ߣ���c��=$\frac{cV{\;}_{��}}{V{\;}_{��}}$��֪���ⶨ��Ũ��ƫ�ߣ���Dѡ��
E��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ���������Ũ�Ƚ��ͣ�����������������������࣬��c��=$\frac{cV{\;}_{��}}{V{\;}_{��}}$��֪���ⶨ��Ũ��ƫ�ߣ���Eѡ��
�ʴ�Ϊ��ADE��
���� ���⿼���к͵ζ���Ϊ��Ƶ���㣬���յζ�ԭ����ʵ��������������Ϊ���Ĺؼ���ע�����������ü��㹫ʽ���ᡢ����������Ŀ�ѶȲ���

| �۵�/�� | �е�/�� | |
| 1-���� | -89.53 | 117.25 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
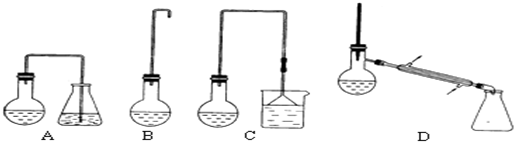
��1���Ʊ�1-�嶡���װ��Ӧѡ����ͼ�е�C������ţ�����Ӧ����ʱ���¶Ȳ��˳���100�棬�����Ƿ�ֹ1-�嶡�����������ݳ���Ӱ��������¶�̫�ߣ�Ũ������������ǿ�����������廯�⣮
��2���Ʊ������У������Ũ������廯�Ƶ������Ƕ��߷�Ӧ����HBr��
��3����Ӧ��������Ӧ�������1-�嶡����������Ӧѡ�õ�װ����D��������ţ����ò���Ӧ���Ƶ��¶ȣ�t����Χ��101.6���t��117.25�森
��4������ȥ������е���������Br2���������������ʺϵ���c��������ĸ��
a��NaI b��NaOH c��Na2SO3 d��KCl��
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
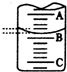
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ25.40mL����ʱ�ζ�����Һ����� ������24.60ml��
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
A��ʵ�����ʱ���ӵζ�����Һ�棬��ȡ�ζ��յ�ʱNaOH��Һ�����
B���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
C��ʢװ��Һ�ĵζ���װҺǰ������ˮϴ�ӣ�δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
��4�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ�����ػ���c��CH3COOH��=$\frac{c����25.35+25.30��}{2V}$mol/L��
| A�� | �����巢��װ���ϵ�ȼ����������ʱ�������ȼ�������Ĵ��� | |
| B�� | �������պ�����������ʵ��������ȴ������ | |
| C�� | ����С�Ĵƾ���ʹ�ƾ��Ż�ʱ��Ӧ��ʪĨ������ | |
| D�� | ������������У����������ӷ�ʯ��Ӧ����ֹͣ���ȣ�����ƿ��ȴ���ټ����ʯ |
| A�� | ���ȷ�Ӧ�ڳ����¶��������� | |
| B�� | ���ȷ�Ӧ�����ȾͲ��ᷢ�� | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ�������ȷ�Ӧ | |
| D�� | ���ݷ�Ӧ����������������������Դ�С��ȷ����Ӧ�� |
| A�� | ���� | B�� | ���ȼ� | C�� | ������ | D�� | ����� |
| A�� | ��һ��Ԫ����ɵ�����һ���ǵ��� | |
| B�� | �����ܵ����H+�Ļ����ﶼ���� | |
| C�� | ���ܵ����OH-��ʹ��Һ�Լ��ԣ�����Һ�Լ��ԵIJ�һ���Ǽ� | |
| D�� | ������ˮ�γɵ���Һ�ܵ��磬�����ǵ���� |
 ����ͬѧ���ø��������ȡ���������ռ��Ͳ��������������װ����ͼ��ʾ��
����ͬѧ���ø��������ȡ���������ռ��Ͳ��������������װ����ͼ��ʾ�� ��1.0mol I2 ��g����2.4mcl H2����ij2L�ܱ������У���ijһ�¶��·�����ӦH2��g��+I2��g��?2HI��g����H��0������ƽ�⣬12��ת����a��I2����ʱ��仯��ͼ���ߢ���ʾ
��1.0mol I2 ��g����2.4mcl H2����ij2L�ܱ������У���ijһ�¶��·�����ӦH2��g��+I2��g��?2HI��g����H��0������ƽ�⣬12��ת����a��I2����ʱ��仯��ͼ���ߢ���ʾ