��Ŀ����
ij��Һ�п��ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Al3+��K+����������ʵ�飺
��1��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.01 mol ���壬ͬʱ�������ɫ���������ˣ�������ϴ�ӣ����գ��õ�0.8 g���壻
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g������
�ɴ˿�֪ԭ��Һ�У�������
��1��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.01 mol ���壬ͬʱ�������ɫ���������ˣ�������ϴ�ӣ����գ��õ�0.8 g���壻
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g������
�ɴ˿�֪ԭ��Һ�У�������
| A�����ٴ���CO32-��Cl-�е�һ�� |
| B��Cl-һ�����ڣ�K+���ܴ��� |
| C��Cl-һ�����ڣ���c��Cl-����0.6mol?L-1 |
| D����Һ�����ٴ���4������ |
���㣺���ӷ���ʽ���йؼ���,�������ӵļ��鷽��
ר�⣺
��������1��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.01 mol���壬ͬʱ�������ɫ������˵����Һ�к���NH4+��Fe3+���������ӹ���֪����Һ��һ������CO32-������ԭ���غ��n��NH3��=n��NH4+��=0.01mol��
����ΪFe��OH��3�����ˣ�������ϴ�ӣ����գ����յõ��Ĺ�����Fe2O3��n��Fe2O3��=
=0.005mol������Feԭ���غ��n��Fe3+��=2n��Fe2O3��=0.01mol��
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������˵��ԭ����Һ�к���Al3+���õ��ij�����Al��OH��3������Alԭ���غ��n��Al3+��=n[Al��OH��3]=0.01mol��
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g����������ΪBaSO4����ԭ����Һ�к���SO42-��n��BaSO4��=n��SO42-��=
=0.01mol��
��Һ�ʵ����ԣ��������������������ȣ�3n��Fe3+��+3n��Al3+��+n��NH4+��=0.03mol+0.03mol+0.01mol=0.07mol��2n��SO42-��=0.02mol��������Һ�л�����Cl-��������֪������ȷ���Ƿ���K+���ݴ˷������
����ΪFe��OH��3�����ˣ�������ϴ�ӣ����գ����յõ��Ĺ�����Fe2O3��n��Fe2O3��=
| 0.8g |
| 160g/mol |
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������˵��ԭ����Һ�к���Al3+���õ��ij�����Al��OH��3������Alԭ���غ��n��Al3+��=n[Al��OH��3]=0.01mol��
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g����������ΪBaSO4����ԭ����Һ�к���SO42-��n��BaSO4��=n��SO42-��=
| 2.33g |
| 233g/mol |
��Һ�ʵ����ԣ��������������������ȣ�3n��Fe3+��+3n��Al3+��+n��NH4+��=0.03mol+0.03mol+0.01mol=0.07mol��2n��SO42-��=0.02mol��������Һ�л�����Cl-��������֪������ȷ���Ƿ���K+���ݴ˷������
���
�⣺��1��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.01 mol���壬ͬʱ�������ɫ������˵����Һ�к���NH4+��Fe3+���������ӹ���֪����Һ��һ������CO32-������ԭ���غ��n��NH3��=n��NH4+��=0.01mol��
����ΪFe��OH��3�����ˣ�������ϴ�ӣ����գ����յõ��Ĺ�����Fe2O3��n��Fe2O3��=
=0.005mol������Feԭ���غ��n��Fe3+��=2n��Fe2O3��=0.01mol��
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������˵��ԭ����Һ�к���Al3+���õ��ij�����Al��OH��3������Alԭ���غ��n��Al3+��=n[Al��OH��3]=0.01mol��
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g����������ΪBaSO4����ԭ����Һ�к���SO42-��n��BaSO4��=n��SO42-��=
=0.01mol��
��Һ�ʵ����ԣ��������������������ȣ�3n��Fe3+��+3n��Al3+��+n��NH4+��=0.03mol+0.03mol+0.01mol=0.07mol��2n��SO42-��=0.02mol��������Һ�л�����Cl-��������֪������ȷ���Ƿ���K+��
A��ͨ�����Ϸ���֪��ԭ��Һ�в�����CO32-��һ������Cl-����A����
B��ͨ�����Ϸ���֪��ԭ��Һ��Cl-һ�����ڣ�K+���ܴ��ڣ���B��ȷ��
C��Cl-һ�����ڣ������Һ��û��K+��c��Cl-��=0.5mol/L���������K+��c��Cl-����0.5mol/L������ԭ��Һ��c��Cl-����0.5mol?L-1����C����
D����Һ�����ٴ���Cl-��SO42-��NH4+��Fe3+��Al3+�������ӣ���D����
��ѡB��
����ΪFe��OH��3�����ˣ�������ϴ�ӣ����գ����յõ��Ĺ�����Fe2O3��n��Fe2O3��=
| 0.8g |
| 160g/mol |
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������˵��ԭ����Һ�к���Al3+���õ��ij�����Al��OH��3������Alԭ���غ��n��Al3+��=n[Al��OH��3]=0.01mol��
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g����������ΪBaSO4����ԭ����Һ�к���SO42-��n��BaSO4��=n��SO42-��=
| 2.33g |
| 233g/mol |
��Һ�ʵ����ԣ��������������������ȣ�3n��Fe3+��+3n��Al3+��+n��NH4+��=0.03mol+0.03mol+0.01mol=0.07mol��2n��SO42-��=0.02mol��������Һ�л�����Cl-��������֪������ȷ���Ƿ���K+��
A��ͨ�����Ϸ���֪��ԭ��Һ�в�����CO32-��һ������Cl-����A����
B��ͨ�����Ϸ���֪��ԭ��Һ��Cl-һ�����ڣ�K+���ܴ��ڣ���B��ȷ��
C��Cl-һ�����ڣ������Һ��û��K+��c��Cl-��=0.5mol/L���������K+��c��Cl-����0.5mol/L������ԭ��Һ��c��Cl-����0.5mol?L-1����C����
D����Һ�����ٴ���Cl-��SO42-��NH4+��Fe3+��Al3+�������ӣ���D����
��ѡB��
���������⿼�����ӹ��桢���ӷ���ʽ���йؼ��㣬���ؿ���������⡢������������ȷ����֮��ķ�Ӧ�����ӹ��������ǽⱾ��ؼ���ע�����غ�����ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�����ж���ȷ���ǣ�������
| A����ij��Һ������ɫ��Ӧʱ������ɫΪ��ɫ�������Һ��һ����Na+��������K+ |
| B����ij��Һ�м���AgNO3��Һ������ɫ����������ϴ����ʱ�������ܽ⣬��ȷ����Cl-���� |
| C����������ʱ����ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ����CO32-���� |
| D���ֱ���Mg2+��Cu2+��Fe2+��Na+�������ӵ���Һ��ֻ��NaOH��Һ�����ܼ���ɹ� |
������ɫ��Ӧ����K+ʱ�������²��裬������ȷ�IJ���˳���ǣ�������
��պȡ����Һ �����ھƾ��ƻ��������� ������ɫ�ܲ����۲� ����ϡ����ϴ����˿��
��պȡ����Һ �����ھƾ��ƻ��������� ������ɫ�ܲ����۲� ����ϡ����ϴ����˿��
| A���ܢڢ٢ڢ� | B���٢ڢۢ� |
| C���ܢ٢ڢ� | D���ڢ٢ۢ� |
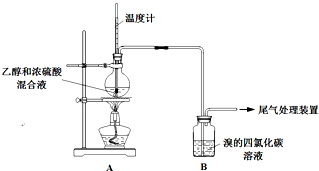 ʵ��������ϩʱ��������������ʹBr2 �����Ȼ�̼��Һ��ɫ���ס���ͬѧ����ͼʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���
ʵ��������ϩʱ��������������ʹBr2 �����Ȼ�̼��Һ��ɫ���ס���ͬѧ����ͼʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���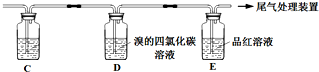

 ����A��һ�ְ�ɫ���壬����ŨNaOH��Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C����Բ����ƿ�ռ������C������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��
����A��һ�ְ�ɫ���壬����ŨNaOH��Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C����Բ����ƿ�ռ������C������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��