��Ŀ����
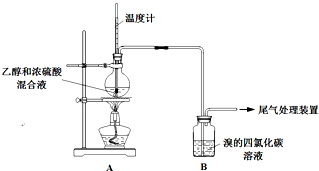 ʵ��������ϩʱ��������������ʹBr2 �����Ȼ�̼��Һ��ɫ���ס���ͬѧ����ͼʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���
ʵ��������ϩʱ��������������ʹBr2 �����Ȼ�̼��Һ��ɫ���ס���ͬѧ����ͼʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���ʵ�����������
| �� �� | �� �� |
| ��ȼ�ƾ��ƣ� ������170�� | ��A����ƿ��Һ�彥����� ��B����������ð������Һ����ɫ |
| �� | |
| ʵ����ϣ� ��ϴ��ƿ | ��A����ƿ�ڸ���������ɫ����״��д̼�����ζ�ݳ� |
��2����Һ��������ڡ���˵��Ũ�������
��3������ʹB����Һ��ɫ�����ʣ�����Ϊ��C2H4������Ϊ�����ų�SO2�����ã�
�ٸ��ݼĹ۵㣬ʹB����Һ��ɫ��Ӧ�Ļ�ѧ����ʽ��
���Ҹ����������Ϊ������SO2����B����SO2 ��Ӧʹ��Һ��ɫ��������
��Ϊ֤ʵ���Թ۵㣬�ס�������ʵ�飬��������������
| �� �� | �� �� | |
| �� | ��A��B������һ��װ��ij���Լ���ϴ��ƿ | Br2��CCl4��Һ��ɫ |
�� | ��A���ӵ�װ�����£�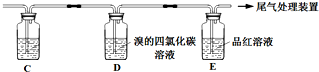 | D����Һ�ɺ���ɫ��Ϊdz����ɫʱ��E����Һ��ɫ |
b�������ҵ���ƣ�C��ʢ�ŵ��Լ���
c����Ϊ��һ����֤��۵㣬ȡ����D����Һ�����뼸��BaCl2��Һ��������������ɫ������dz����ɫ��ʧ��������Ӧ�����ӷ���ʽ��
��4������ʵ��õ��Ľ�����
���㣺����ʵ�鷽�������
ר�⣺ʵ�������
��������1���Ҵ�����һ���������·�����ȥ��Ӧ������ϩ��
��2��Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԣ�
��3������ϩ������ˮ�����ӳɷ�Ӧ��
���嵥�ʿ��ԺͶ���������������ԭ��Ӧ��
��a���������ƿ��ԺͶ�����������Ӧ��
b��Ũ���������ˮ�ԣ�����������������ܽ���ϩ������
c���嵥�ʿ��ԺͶ���������������ԭ��Ӧ����������Ӻͱ����ӷ�Ӧ�����ɰ�ɫ������
��4����ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ������������嵥�ʵķ�Ӧ������ˮ��Һ�н��У�
��2��Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԣ�
��3������ϩ������ˮ�����ӳɷ�Ӧ��
���嵥�ʿ��ԺͶ���������������ԭ��Ӧ��
��a���������ƿ��ԺͶ�����������Ӧ��
b��Ũ���������ˮ�ԣ�����������������ܽ���ϩ������
c���嵥�ʿ��ԺͶ���������������ԭ��Ӧ����������Ӻͱ����ӷ�Ӧ�����ɰ�ɫ������
��4����ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ������������嵥�ʵķ�Ӧ������ˮ��Һ�н��У�
���
�⣺��1���Ҵ�����Ũ���Ტ���ȵ������·�����ȥ��Ӧ������ϩ��ԭ������ʽΪ��CH3CH2OH
CH2�TCH2��+H2O���ʴ�Ϊ��CH3CH2OH
CH2�TCH2��+H2O��
��2��Ũ���������ˮ�ԣ���ʹ�Ҵ���Һ��������ڡ����ʴ�Ϊ����ˮ�ԣ�
��3���ٸ��ݼĹ۵㣬��ˮ����ʹB����Һ��ɫ����Ӧ�Ļ�ѧ����ʽ��CH2=CH2+Br2��CH2Br-CH2Br���ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
���Ҹ����������Ϊ������SO2����B���嵥�ʿ��ԺͶ���������������ԭ��Ӧ���ʴ�Ϊ��H2O��Br2��
��a�����ݼ���ƣ�ϴ��ƿ��ʢ�ŵ��Լ����������ƣ����ԺͶ�������Ӧ�����ն��������ų���������ĸ��ţ��ʴ�Ϊ��NaOH ��Һ��
b�������ҵ���ƣ�C��ʢ�ŵ��Լ��ǣ�Ũ���������ˮ�ԣ���������������ɽ���ϩ����Ϊ������̼���ų��ڼ����������ʱ��ϩ�ĸ��ţ��ʴ�Ϊ��Ũ���
c���嵥�ʿ��ԺͶ���������������ԭ��Ӧ��������������Ӻ������ӣ���������Ӻͱ����ӷ�Ӧ�����ɰ�ɫ��������ط�Ӧ�����ӷ���ʽΪSO2+2H2O+Br2�T4H++2Br-+SO42-��SO42-+Ba2+�TBaSO4����SO2+2H2O+Br2+Ba2+�T4H++2Br-+BaSO4����
�ʴ�Ϊ��SO2+2H2O+Br2�T4H++2Br-+SO42-��SO42-+Ba2+�TBaSO4����SO2+2H2O+Br2+Ba2+�T4H++2Br-+BaSO4����
��4������ʵ����̿���֪������ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ������������嵥�ʵķ�Ӧ������ˮ��Һ�н��У�
�ʴ�Ϊ����ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ�������SO2����ʹBr2�����Ȼ�̼��Һ��ɫ��
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
��2��Ũ���������ˮ�ԣ���ʹ�Ҵ���Һ��������ڡ����ʴ�Ϊ����ˮ�ԣ�
��3���ٸ��ݼĹ۵㣬��ˮ����ʹB����Һ��ɫ����Ӧ�Ļ�ѧ����ʽ��CH2=CH2+Br2��CH2Br-CH2Br���ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
���Ҹ����������Ϊ������SO2����B���嵥�ʿ��ԺͶ���������������ԭ��Ӧ���ʴ�Ϊ��H2O��Br2��
��a�����ݼ���ƣ�ϴ��ƿ��ʢ�ŵ��Լ����������ƣ����ԺͶ�������Ӧ�����ն��������ų���������ĸ��ţ��ʴ�Ϊ��NaOH ��Һ��
b�������ҵ���ƣ�C��ʢ�ŵ��Լ��ǣ�Ũ���������ˮ�ԣ���������������ɽ���ϩ����Ϊ������̼���ų��ڼ����������ʱ��ϩ�ĸ��ţ��ʴ�Ϊ��Ũ���
c���嵥�ʿ��ԺͶ���������������ԭ��Ӧ��������������Ӻ������ӣ���������Ӻͱ����ӷ�Ӧ�����ɰ�ɫ��������ط�Ӧ�����ӷ���ʽΪSO2+2H2O+Br2�T4H++2Br-+SO42-��SO42-+Ba2+�TBaSO4����SO2+2H2O+Br2+Ba2+�T4H++2Br-+BaSO4����
�ʴ�Ϊ��SO2+2H2O+Br2�T4H++2Br-+SO42-��SO42-+Ba2+�TBaSO4����SO2+2H2O+Br2+Ba2+�T4H++2Br-+BaSO4����
��4������ʵ����̿���֪������ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ������������嵥�ʵķ�Ӧ������ˮ��Һ�н��У�
�ʴ�Ϊ����ϩ��ʹBr2�����Ȼ�̼��Һ��ɫ�������SO2����ʹBr2�����Ȼ�̼��Һ��ɫ��
���������⿼��ѧ���Ҵ��Ļ�ѧ���ʣ����ʵ�鿼����������Ŀ���Ѷȣ��ۺ��Խ�ǿ��Ҫ��ѧ�����з����ͽ�������������

��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
�����Ŀ
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A����������Һ�еμӹ���Ũ��ˮ��Al3++4OH-�TAlO2-+2H2O |
| B���ù���������ữ�ĺ����ҽ���Һ����ȡ�⣺2I-+H2O2+2H+=I2+2H2O |
| C��̼�������Һ�м��������ռ���Һ��HCO3-+OH-=CO32-+H2O |
| D���������ƹ�����ˮ��Ӧ��2O22-+2H2O=4OH-+O2�� |
ij��Һ�п��ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Al3+��K+����������ʵ�飺
��1��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.01 mol ���壬ͬʱ�������ɫ���������ˣ�������ϴ�ӣ����գ��õ�0.8 g���壻
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g������
�ɴ˿�֪ԭ��Һ�У�������
��1��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0.01 mol ���壬ͬʱ�������ɫ���������ˣ�������ϴ�ӣ����գ��õ�0.8 g���壻
��2����1������Һ��ͨ������CO2�����ˣ��õ�0.01 mol������
��3����2������Һ�м��������������ữ��BaCl2��Һ���õ�2.33g������
�ɴ˿�֪ԭ��Һ�У�������
| A�����ٴ���CO32-��Cl-�е�һ�� |
| B��Cl-һ�����ڣ�K+���ܴ��� |
| C��Cl-һ�����ڣ���c��Cl-����0.6mol?L-1 |
| D����Һ�����ٴ���4������ |


 �����ƺ������Ʒֱ���ˮ��Ӧ�кܶ�ֵ��̽�������⣮
�����ƺ������Ʒֱ���ˮ��Ӧ�кܶ�ֵ��̽�������⣮

