��Ŀ����
16��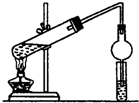 ������ĿҪ�ش��������⣺
������ĿҪ�ش��������⣺��1��ijͬѧ���Ҵ��������Ũ������ȡ����������װ����ͼ��ʾ���÷�Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH
 CH3COOC2H5+H2O��װ���и���ܵ������Ƿ�ֹ������С�Թ��е��Լ�Ϊ����̼������Һ��
CH3COOC2H5+H2O��װ���и���ܵ������Ƿ�ֹ������С�Թ��е��Լ�Ϊ����̼������Һ����2��ij������A����Է�������Ϊ104������̼����������Ϊ92.3%����A�Ľṹ��ʽΪ
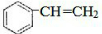 ����һ�������£�A�����ۺϷ�Ӧ�õ�һ�ָ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ
����һ�������£�A�����ۺϷ�Ӧ�õ�һ�ָ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ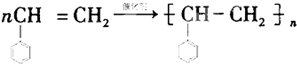 ��A��������ȫ�ӳɺ�����һ�ȴ��ﹲ��5�֣�
��A��������ȫ�ӳɺ�����һ�ȴ��ﹲ��5�֣�
���� ��1��������Ҵ���Ũ���������¼�����������������������������ע���ֹ�������Դ˽����⣻
��2��������Է��������ͺ�̼���ɼ��㺬��������������C��Hԭ����Ŀ����֪����ʽ�������ʺ��б������ܷ����ۺϷ�Ӧ�õ�һ�ָ߷��ӻ����˵��A����C=C�����ݷ����е�Ч��ԭ����Ŀ�жϣ�
��� �⣺��1���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH CH3COOC2H5+H2O�����������л����Ҵ������ᣬ����������ˮ�������ܺ�̼���Ʒ�Ӧ���ײ�����������ʵ����ʹ�����ιܣ����β��ֿռ��Һ������ʱ�ɻ��壬�����ܷ�ֹ�������Ʊ���������ʱ���ñ���̼������Һ��������������Ŀ���dz�ȥ�Ҵ������ᡢ���������������ܽ�ȣ����ڷֲ㣻
CH3COOC2H5+H2O�����������л����Ҵ������ᣬ����������ˮ�������ܺ�̼���Ʒ�Ӧ���ײ�����������ʵ����ʹ�����ιܣ����β��ֿռ��Һ������ʱ�ɻ��壬�����ܷ�ֹ�������Ʊ���������ʱ���ñ���̼������Һ��������������Ŀ���dz�ȥ�Ҵ������ᡢ���������������ܽ�ȣ����ڷֲ㣻
�ʴ�Ϊ��CH3COOH+CH3CH2OH CH3COOC2H5+H2O����ֹ����������̼������Һ��
CH3COOC2H5+H2O����ֹ����������̼������Һ��
��2��1molA��n��C��=$\frac{104g��92.3%}{12g/mol}$=8mol��n��H��=$\frac{104g����1-92.3%��\\;1g/mol\\;}{1g/mol}$=8�������ʽΪC8H8���ܷ����ۺϷ�Ӧ�õ�һ�ָ߷��ӻ����˵��A����C=C�����ԽṹΪ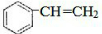 ������ϩ�ڴ��������·����ӳɷ�Ӧ���ɾ۱���ϩ����Ӧ�Ļ�ѧ����ʽΪ��
������ϩ�ڴ��������·����ӳɷ�Ӧ���ɾ۱���ϩ����Ӧ�Ļ�ѧ����ʽΪ�� ��A��������ȫ�ӳɺ����Ϊ�ұ����ұ��Ľṹ��ʽΪ��
��A��������ȫ�ӳɺ����Ϊ�ұ����ұ��Ľṹ��ʽΪ��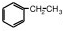 �������к���5�ֵ�ЧHԭ�ӣ���һ�ȴ�����5�֣�
�������к���5�ֵ�ЧHԭ�ӣ���һ�ȴ�����5�֣�
�ʴ�Ϊ��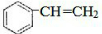 ��
�� ��5��
��5��
���� ���⿼���������������Ʊ����л������ʽ��ȷ����ͬ���칹����Ŀ�����㣬��Ŀ�ѶȲ���ע������ͬ���칹��ĸ����дԭ���ؼ�����ȷ�ж��л�������к��е�Ч��ԭ����Ŀ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �ֱ����⻯����Һ���۲���ɫ�仯 | |
| B�� | �ֱ���ȣ��۲��Ƿ����ɳ��� | |
| C�� | �ֱ�μ�Ũ���� | |
| D�� | �ֱ����գ���ζ�� |
 ʵ������ȡ������������Ҫ��������
ʵ������ȡ������������Ҫ������������25mL�Ĵ��Թ�A�а������2��3��2�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10min��
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���÷ֲ㣮
�ܷ�������������㡢ϴ�ӡ����
�������ݿɹ��ο���
| ���� | �Ҵ� | ���� | �������� | Ũ���� |
| �ۣ��棩 | -117.0 | 16.6 | -83.6 | ------ |
| �У��棩 | 78.0 | 117.9 | 77.5 | 338.0 |
��1�����Ƹû��Һ�Լ�������˳��Ϊ���ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ��ȴ���ټӱ����ᣮ
��2��д���÷�Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3CH2OH
 CH3COOC2H5+H2O��
CH3COOC2H5+H2O����3���÷�Ӧ����Щ��ʩ������������ת����CD��
A��ʹ�����Ƭ B���ƾ��Ƽ���C��Ũ��������ˮ�� D�������ɵ�������������
��4������ʵ���б���̼������Һ����Ҫ��BCD������ĸ��
A�������������ɣ���������
B�����������������ܽ�ȣ�����Һ��ֲ�
C���������������
D���������������Ҵ�
��5�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ����¶ȹ��߿��ܷ�����������Ӧ�ڼ��������Ҵ��Ļӷ���
��6������������������һ���ñ���ʳ��ˮ�ͱ����Ȼ�����Һϴ�ӣ�ͨ��ϴ�ӳ�ȥ̼���ơ��Ҵ���Ϊ�˸�������������ѡ�õĸ����ΪB������ĸ����
A��Ũ���� B����ˮNa2SO4 C����ʯ�� D��NaOH���壮
| A�� | a�������Է�������һ����b�������Է��������� | |
| B�� | a���������CO��b���������CH4 | |
| C�� | A�����������������������B������������������� | |
| D�� | ������A���������һ������B��������� |
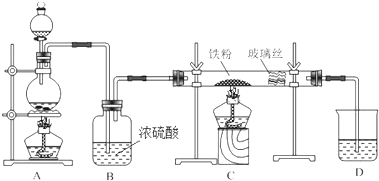
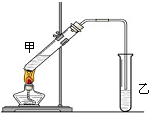 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺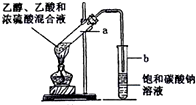 ��ͼΪʵ������ȡ����������װ�ã�
��ͼΪʵ������ȡ����������װ�ã� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O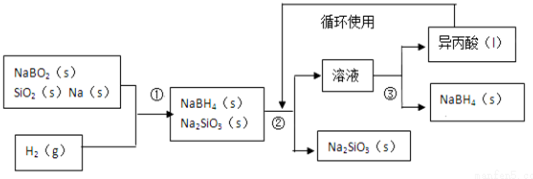
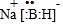 ������BԪ�صĻ��ϼ�Ϊ+3
������BԪ�صĻ��ϼ�Ϊ+3