��Ŀ����
6��ͬ��ͬѹ�£���A��B������ȫ��ͬ�������������Բ��ƣ���A�����г���a���壬B�����г���b���壬������������������ȣ�A�������ڵ����У�����ֹ������B���������£�N2H4�������У����������������й���������ȷ���ǣ�������| A�� | a�������Է�������һ����b�������Է��������� | |
| B�� | a���������CO��b���������CH4 | |
| C�� | A�����������������������B������������������� | |
| D�� | ������A���������һ������B��������� |
���� A������ڵ����о�ֹ������B��������£�N2H4�����ϸ���˵��A������ܶȺ͵������ܶȽӽ���B���ܶ�С���£�N2H4�������ݦ�=$\frac{M}{{V}_{m}}$��֪��ͬ��ͬѹ�£��ܶ���Ħ�����������ȣ�����A����Է��������뵪�������ƣ��ӽ�28��B����Է�������С���£�N2H4������С��32���ݴ˷������
��� �⣺A������ڵ����о�ֹ������B��������£�N2H4�����ϸ���˵��A������ܶȺ͵������ܶȽӽ���B���ܶ�С���£�N2H4�������ݦ�=$\frac{M}{{V}_{m}}$��֪��ͬ��ͬѹ�£��ܶ���Ħ�����������ȣ�����A����Է��������뵪�������ƣ��ӽ�28��B����Է�������С���£�N2H4������С��32��
A�����ݷ�����֪��a�������Է�������һ����b�������Է�������С����A����
B��a����Է�������ԼΪ28������ΪCO��b�������Է�������С��32������Ϊ���飬��B��ȷ��
C��A��B������������������ȣ�������ͬ�����£�����ߺ�����������ʵ�����ȣ���C����
D��a��b����������ʵ�����ȣ�B�������Ħ�������ϴ��������A���������һ��С��B�������������D����
��ѡB��
���� ���⿼�������ʵ����ļ��㣬��Ŀ�ѶȲ�����ȷ���ʵ�����Ħ������������Ħ�����������٤�������Ĺ�ϵ���ɽ������������ѧ���ķ������������Ӧ��������

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±�������������Ũ��Ϊ��0.01mol/L��
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��1��д������������Co2O3������Ӧ�����ӷ���ʽCo2O3+SO32-+4H+=2Co2++SO42-+2H2O��
��2��д��NaClO3������Ӧ����Ҫ���ӷ���ʽClO3-+6Fe2++6H+=Cl-+6Fe3++3H2O������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽClO3-+5Cl-+6H+=3Cl2��+3H2O��
��3������Na2CO3��pH��a�����������õ��ij����ɷ�ΪFe��OH��3��Al��OH��3��
��4��������1���а���3������ʵ���������������������Ũ������ȴ�ᾧ���ˣ��Ƶõ�CoCl2•6H2O�ں��ʱ���ѹ��ɵ�ԭ���ǽ��ͺ���¶ȣ���ֹ��Ʒ�ֽ⣮
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2������Һ���м�����ȡ����Ŀ���dz�ȥ��Һ�е�Mn2+����ʹ�õ����pH��Χ��B��
A��2.0��2.5 B��3.0��3.5
C��4.0��4.5 D��5.0��5.5
��6��Ϊ�ⶨ�ֲ�Ʒ��CoCl2•6H2O��������ȡһ�������Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2•6H2O��������������100%����ԭ������Ǵֲ�Ʒ���п������Ȼ������ʧȥ�˲��ֽᾧˮ������һ�����ɣ�

����������������������ʽ��ȫ����ʱ��Һ��pH���±�����ش��������⣺
| ������ | Fe3+ | Al3+ | Mg2+ |
| pH | 3.2 | 5.2 | 12.4 |
��2������I����Ҫ�ɷ���Fe��OH��3��Al��OH��3��
��3������Һ���пɻ������õ���Ҫ������Na2SO4��
��4������MgCl2��Һ�������ɵõ��Ĺ�����Mg��OH��2����Ҫ�õ�MgCl2Ӧ��ȡ�IJ�������HCl�������н�MgCl2��Һ�������ɣ�
��5����ϡ�������ʱ�γ����������ӷ���ʽ��MgSiO3+2H+=H2SiO3+Mg2+��CaMg��CO3��2+SO42-+4H+=CaSO4+Mg2++2CO2��+2H2O��
| A�� | ������ʹ��ϡ��Һ | B�� | ѡ����ʵĴ��� | ||
| C�� | ��ϸ���巴Ӧ�� | D�� | ��߷�Ӧ��ϵ���¶� |
| A�� | CaCl2$\stackrel{CO_{2}}{��}$CaCO3$\stackrel{����}{��}$CaO | B�� | Fe$\stackrel{����Cl_{2}}{��}$FeCl2$\stackrel{NaOH��Һ}{��}$Fe��OH��2 | ||
| C�� | Al2O3$\stackrel{NaOH��Һ}{��}$NaAlO2$\stackrel{CO_{2}}{��}$Al��OH��3 | D�� | SiO2$\stackrel{ˮ}{��}$H2SiO3$\stackrel{NaOH��Һ}{��}$Na2SiO3 |
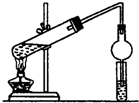 ������ĿҪ�ش��������⣺
������ĿҪ�ش��������⣺ CH3COOC2H5+H2O��װ���и���ܵ������Ƿ�ֹ������С�Թ��е��Լ�Ϊ����̼������Һ��
CH3COOC2H5+H2O��װ���и���ܵ������Ƿ�ֹ������С�Թ��е��Լ�Ϊ����̼������Һ��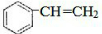 ����һ�������£�A�����ۺϷ�Ӧ�õ�һ�ָ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ
����һ�������£�A�����ۺϷ�Ӧ�õ�һ�ָ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽΪ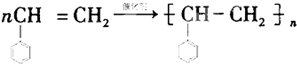 ��A��������ȫ�ӳɺ�����һ�ȴ��ﹲ��5�֣�
��A��������ȫ�ӳɺ�����һ�ȴ��ﹲ��5�֣�