��Ŀ����
1���������������Ҫ�Ļ�ԭ��������������β����������ýȾ����Ư���ȣ������ؾ�ʯ�����еĸ���ƷҺ̬���ƣ�Na2S��������Na2SO3��Na2CO3�����������ʣ�������������ƵĹ����������£�
�ش��������⣺
��1����Ӧ����Na2S��S�����ʵ���֮��1��1��Ӧ�����ӷ���ʽΪS2-+S=2S2-����Ӧ������ȵ�Ŀ����ʹNa2S��S�ܳ�ַ�Ӧ
��2������ʱ���������[m��MnSO4����m��NiSO4•7H2O��=1��4]����ò�ͬ�¶�����ȫ��������ʱ�������
| ��Һ�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ������ȫ����ʱ��/h | 6 | 5.5 | 5.5 | 6 | 20 | 16 | 11 | 11 |
��3������ʱ����������Ҫ�ɷ�Ϊ��Һ̬����ԭ���е����ʣ�Ũ��ʱ���������Ҵ���Ŀ���Ǽ�СNa2S2O3���ܽ�ȣ�
���� ���ݹ�������ͼ��֪��Na2S��S�ڼ��ȵ�����������Na2S2��Na2S2�������������������е�����������Na2S2O3��ͬʱ�п��ܲ������˳�ȥ���������ʼ�����ҺŨ��ʱ�����Ҵ������Լ�СNa2S2O3���ܽ�ȣ��ᾧ����ˮ�ɵ�Na2S2O3•5H2O��
��1������Ԫ���غ����дNa2S��S�����ʵ���֮��1��1��Ӧ�����ӷ���ʽ����Ӧ������ȵ�Ŀ�ı���Na2S��S�ܳ�ַ�Ӧ��
��2�����ݱ��е����ݿ�֪��������ȫ����ʱ����̵ļ�Ϊ���������˵��¶ȣ��¶ȹ���NiSO4•7H2Oʧȥ�ᾧˮ����ʹ�����Ĵ����ü�����
��3����������ķ�����֪�����ijɷݣ�Ũ��ʱ���������Ҵ����Լ�СNa2S2O3���ܽ�ȣ��ݴ˴��⣻
��� �⣺���ݹ�������ͼ��֪��Na2S��S�ڼ��ȵ�����������Na2S2��Na2S2�������������������е�����������Na2S2O3��ͬʱ�п��ܲ������˳�ȥ���������ʼ�����ҺŨ��ʱ�����Ҵ������Լ�СNa2S2O3���ܽ�ȣ��ᾧ����ˮ�ɵ�Na2S2O3•5H2O��
��1������Ԫ���غ��֪Na2S��S�����ʵ���֮��1��1��Ӧ�����ӷ���ʽΪS2-+S=2S2-����Ӧ������ȵ�Ŀ����ʹNa2S��S�ܳ�ַ�Ӧ��
�ʴ�Ϊ��S2-+S=2S2-��ʹNa2S��S�ܳ�ַ�Ӧ��
��2�����ݱ��е����ݿ�֪��������ȫ����ʱ����̵ļ�Ϊ���������˵��¶ȣ���������Ҫ���¶�Ϊ20�桫30�棬�¶ȹ���NiSO4•7H2Oʧȥ�ᾧˮ����ʹ�����Ĵ����ü�����
�ʴ�Ϊ��20�桫30�棻NiSO4•7H2Oʧȥ�ᾧˮ����ʹ�����Ĵ����ü�����
��3����������ķ�����֪�����ijɷ�Ϊ��Һ̬����ԭ���е����ʣ�Ũ��ʱ���������Ҵ����Լ�СNa2S2O3���ܽ�ȣ�������������ƾ����������
�ʴ�Ϊ����СNa2S2O3���ܽ�ȣ�
���� ���⿼���������Ʊ���̽��ʵ�飬��ȷ�������̷�Ӧ���Լ������ǽ���ؼ�����Ŀ�Ѷ��еȣ�����ʱע��Ƚ���Ϣ�е�������ݲ����ܽ���ɣ�������ؿ���ѧ�����������Ӧ�û���֪ʶ��������

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д�| A�� | C5H10 | B�� | C4H8 | C�� | C2H4 | D�� | C2H4Cl2 |
| A�� | ������������ȼ�� | B�� | ����������ȼ�� | ||
| C�� | ������������ȼ�� | D�� | ϸ��˿��������ȼ�� |
| A�� | ������Ӧ����������������Һ��Na+��K+��CO32-��NO3- | |
| B�� | 0.1mol•L-1��NaOH��Һ��K+��Na+��SO42-��CO32- | |
| C�� | 0.1mol•L-1FeCl3��Һ��K+��NH4+��SO42-��SCN- | |
| D�� | ʹ��ɫʯ����Һ������Һ��Ca2+��Na+��ClO-��NO3- |
| A�� | pH=4��NaHSO3��Һ�У�c��Na+����c��H+����c��HSO3-����c��SO32-����C��OH-�� | |
| B�� | 0.2mol•L-1Na2CO3��Һ��0.1mol•L-1����������ϣ��ڻ����Һ�У�c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| C�� | ���������Ũ�ȵ�HR��Һ��NaOH��Һ��ϣ���Һ��һ�����ڣ�c��Na+����c��R-����c��OH-����c��H+�� | |
| D�� | ���������Ũ�ȵĢ�CH3COONa��NaNO3��NaClO��Һ�������������ڣ��٣��� |
| A�� | Na+��Fe2+��Cl-��NO3-���������ڼ�����Һ�Ժ�ɫ����Һ�й��� | |
| B�� | K+��Mg2+��Cl-��I-����������[H+]=10-12 mol•L-1����Һ�й��� | |
| C�� | NaHSˮ������ӷ���ʽΪ��HS-+H2O?S2-+H3O+ | |
| D�� | NaHCO3�ĵ��뷽��ʽΪ��NaHCO3=Na++HCO3-��HCO3-?H++CO32- |

 ͨ����ȥ��Ӧ�Ʊ���Ļ�ѧ����ʽΪ
ͨ����ȥ��Ӧ�Ʊ���Ļ�ѧ����ʽΪ ��ע����Ӧ��������
��ע����Ӧ�������� ��
�� ��
�� ��H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�������͢��������ʽ��ΪC9H8O���Ҷ��ܷ���������Ӧ�����й��ڢ��͢���˵����ȷ����AB��˫ѡ������ĸ����
��H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�������͢��������ʽ��ΪC9H8O���Ҷ��ܷ���������Ӧ�����й��ڢ��͢���˵����ȷ����AB��˫ѡ������ĸ����
 ��
��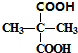 +2C2H5OH$?_{��}^{Ũ����}$
+2C2H5OH$?_{��}^{Ũ����}$ +2H2O��
+2H2O�� ��
�� ��
��