��Ŀ����
2����������λͬѧΪ��֤ij��ɫ����ΪSO2�����е�ʵ����ƣ������ܹ����˵����������SO2���ǣ��������ټ�ͬѧ������ͨ������KMnO4��Һ�У���Һ��ɫ���ٵ���BaCl2��Һ�����ְ�ɫ������
����ͬѧ��ʪ�����ɫʯ����ֽ��������壬��ֽ��죻
�۱�ͬѧ������ͨ��Ʒ����Һ����Һ��ɫ�����Ⱥ��ָֻ���ɫ��
�ܶ�ͬѧ������ͨ����ˮ�У���ˮ��ɫ���ٵμ��ữ���Ȼ�����Һ�а�ɫ�������ɣ�
| A�� | �٢ڢۢ� | B�� | �٢ۢ� | C�� | �ۢ� | D�� | �ڢۢ� |
���� �ټ�ͬѧ������ͨ������KMnO4��Һ�У���Һ��ɫ��˵���������л�ԭ�ԣ��ٵ���BaCl2��Һ�����ְ�ɫ�������˳���ΪBaSO4����֪����ΪSO2��
����ͬѧ��ʪ�����ɫʯ����ֽ��������壬��ֽ��죬˵���������ˮ��Һ�����ԣ�ͨ��SO2��HCl��CO2��H2S�������ˮ��Һ�����ԣ�
�۱�ͬѧ������ͨ��Ʒ����Һ����Һ��ɫ��˵����������Ư���ԣ����Ⱥ��ָֻ���ɫ��˵�����ɵ��Dz��ȶ�����ɫ���ʣ���֪������ΪSO2��
�ܶ�ͬѧ������ͨ����ˮ�У���ˮ��ɫ��˵���������л�ԭ�ԣ��ٵμ��ữ���Ȼ�����Һ�а�ɫ�������ɣ��˳���ΪBaSO4����֪����ΪSO2���ݴ˷�������
��� �⣺�ٽ�SO2����ͨ������KMnO4��Һ�У���Һ��ɫ���ٵ���BaCl2��Һ������BaSO4��ɫ�������ʢ���ȷ��
����ͬѧ��ʪ�����ɫʯ����ֽ��������壬��ֽ��죬���������SO2��HCl��CO2��H2S�ȣ��ʢڴ���
�۽�SO2����ͨ��Ʒ����Һ����Һ��ɫ�����Ⱥ��ָֻ���ɫ���ʢ���ȷ��
�ܽ�SO2����ͨ����ˮ�У���ˮ��ɫ���ٵμ��ữ���Ȼ�����Һ��BaSO4��ɫ�������ɣ��ʢ���ȷ��
��ѡ��B��
���� ����SO2��������ʼ����飬��Ҫ�漰��ԭ�ԡ�Ư���Լ�ˮ��Һ������ԣ����ų���������ʸ����ǽ���ؼ����������飬�ѶȲ���

 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�| A�� | MgCl2���� | B�� | ���ڵ�KOH | C�� | Һ̬HCl | D�� | NaCl��Һ |

| A�� | ��ͼ����ʾʵ��ɱȽ��ȡ�̼��������Ԫ�صķǽ�����ǿ�� | |
| B�� | ��ͼ����ʾʵ��װ���ſ������ռ�NO2���� | |
| C�� | ͼ�۱�ʾ���淴ӦC��s��+H2O��g��?CO��g��+H2��g���ġ�HС��0 | |
| D�� | ͼ������װ����ͨ�����ߵĵ�������ͬʱ�����ĸ������ϵ����ʵ�������ͬ |
 ����ӦIO3-+5I-+6H+?3I2+3H2O��Ƴ���ͼ��ʾ��ԭ��أ��ס����ձ��ж����������Һ����ʼʱ����ձ��м�������Ũ���ᣬ������ָ�뷢��ƫת��һ��ʱ�������ָ��ص��㣬������ձ��е��뼸��ŨNaOH��Һ��������ָ���ٴη���ƫת�������жϲ���ȷ���ǣ�������
����ӦIO3-+5I-+6H+?3I2+3H2O��Ƴ���ͼ��ʾ��ԭ��أ��ס����ձ��ж����������Һ����ʼʱ����ձ��м�������Ũ���ᣬ������ָ�뷢��ƫת��һ��ʱ�������ָ��ص��㣬������ձ��е��뼸��ŨNaOH��Һ��������ָ���ٴη���ƫת�������жϲ���ȷ���ǣ�������| A�� | ���ε�����ָ��ƫת�����෴�������ƶ���Ϊ��ʱ����Ӧ�ﵽ��ѧƽ��״̬ | |
| B�� | ����ʵ���У������е��������ƶ������෴ | |
| C�� | ��ʼ��������Ũ����ʱ��ֻ�����ձ�����Һ���� | |
| D�� | ����ձ��е��뼸��ŨNaOH��Һ������ʯī�缫�Ϸ�����ԭ��Ӧ |
| ������ | K+ NH4+ Fe2+ Mg2+ Al3+ Cu2+ |
| ������ | OH- Cl- Al02- CO32- SiO32-SO42-�� |

����˵����ȷ���ǣ�������
| A�� | ԭ��Һ��ֻ����NH4+ Fe2+Cl- SO42- | |
| B�� | �ɳ���A�ƶ�ԭ��Һ��һ������SO42- | |
| C�� | ��ҺA�п��ܺ���K+ Al3+ Cl- | |
| D�� | ����B��һ������Mg��OH��2 |

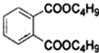 +2NaOH$\stackrel{����}{��}$
+2NaOH$\stackrel{����}{��}$ +2CH3CH2CH2CH2OH��
+2CH3CH2CH2CH2OH��