��Ŀ����
1��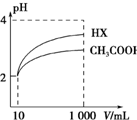 ����HA�ĵ��볣��Ka=$\frac{c��{H}^{+}��•c��{A}^{-}��}{c��HA��}$��25��ʱ����������ĵ��볣�����£�
����HA�ĵ��볣��Ka=$\frac{c��{H}^{+}��•c��{A}^{-}��}{c��HA��}$��25��ʱ����������ĵ��볣�����£�| ���ữѧʽ | HNO2 | CH3COOH | HCN | H2CO3 |
| ���볣�� | 5.1��10-4 | 1.8��10-5 | 6.2��10-10 | K1=4.4��10-7 K2=4.7��10-11 |
�����ʵ���Ũ����ͬ�������ᣬ��pH�ɴ�С��˳����HCN��H2CO3��CH3COOH��HNO2��
�ڷֱ�����������ͬpH��HCl��Һ��CH3COOH��Һ�м���������Zn�ۣ���Ӧ�տ�ʼʱ����H2�����ʣ�v��HCl��=v��CH3COOH�����=����������������ͬ������Ӧ��ȫ������������������m��H2��������m��H2��������
�۽�0.2 mol/L HCN��Һ��0.1 mol/L Na2CO3��Һ�������ϣ�������Ӧ�Ļ�ѧ����ʽΪHCN+Na2CO3�TNaCN+NaHCO3��
��2�������Ϊ10 mL��pH��Ϊ2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1 000 mL��ϡ��������ҺpH�仯��ͼ��ʾ��ϡ�ͺ�HX��Һ��ˮ�����c��H+�� �ȴ�����Һ��ˮ�����c��H+�����볣��Ka��HX��Ka��CH3COOH�������������=����������������ϡ����ͬ��������ǿ����pH�仯�ϴ�ǿ������볣���ϴ�ͼ�п���HX��pH�仯�ϴ�
���� ��1��������ĵ���ƽ�ⳣ��Խ��������Խǿ��
��pH��ͬ�IJ�ͬ�����У�������Ũ����ͬ�����������Ũ�ȴ���������Ũ�ȣ����������Ũ�ȵ���������Ũ�ȣ�
�۸������Թ�ϵ�жϣ�
��2��ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
��� �⣺��1���پݵ���ƽ�ⳣ���Ĵ�С����������ƽ�ⳣ��Խ��������Խǿ�����ԣ�HCN��H2CO3��CH3COOH��HNO2������Խǿ����pHԽС������pH��ϵΪ��HCN��H2CO3��CH3COOH��HNO2��
�ʴ�Ϊ��HCN��H2CO3��CH3COOH��HNO2��
��pH��ͬ�IJ�ͬ�����У�������Ũ����ͬ����Zn��Ӧ������ͬ�����������Ũ�ȴ���������Ũ�ȣ����������Ũ�ȵ���������Ũ�ȣ����Դ����Ũ�ȴ���HCl��Ũ�ȣ���������������������m��H2��������m��H2��������
�ʴ�Ϊ��=������
���ɵ��볣����֪�����ԣ�H2CO3��HCN��CO3-����0.2 mol/L HCN��Һ��0.1 mol/L Na2CO3��Һ�������ϣ�������Ӧ�Ļ�ѧ����ʽΪHCN+Na2CO3�TNaCN+NaHCO3��
�ʴ�Ϊ��HCN+Na2CO3�TNaCN+NaHCO3��
��2����ͼ��֪��ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С������HX��Һ��ˮ���������c��H+����ϡ����ͬ��������ǿ����pH�仯�ϴ�ǿ������볣���ϴ���ͼ��֪ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
�ʴ�Ϊ������ϡ����ͬ��������ǿ����pH�仯�ϴ�ǿ������볣���ϴ�ͼ�п���HX��pH�仯�ϴ�
���� ���⿼����������ʵĵ��볣��������ˮ�⼰����ԵıȽϡ�pH�����ϡ�͵�֪ʶ��ע��ˮ�������Խ��Խˮ���ϡ����ǿ�ı仯������������ۺ��Խϴ���Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ���������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Rb��K��Na��Li | B�� | Cl��S��P��N | ||
| C�� | Na+��Mg2+��Al3+��H+ | D�� | I-��Br-��Cl-��F- |
| A�� | ��ͭ��[Cu2��OH��2CO3]��ͭ�����������н��ݣ���ȥͭ�� | |
| B�� | �����½�ͭ˿����ʢ�������ļ���ƿ�У��۲�CuCl2������ | |
| C�� | �����Ʒ�������ͭ��Һ�п����û�������ͭ | |
| D�� | ��ͭ˿����Ũ������ȣ���Ӧ���ˮ���뷴Ӧ���У��۲�����ͭ��Һ����ɫ������ |
 �л�����ؾ��в����ؽ���������Դ�����ٵ��ŵ��������Ŀ��ijPYT-﮵���ܷ�Ӧ��ͼ��������������ȷ���ǣ�������
�л�����ؾ��в����ؽ���������Դ�����ٵ��ŵ��������Ŀ��ijPYT-﮵���ܷ�Ӧ��ͼ��������������ȷ���ǣ�������| A�� | PYT����ʽΪC16H6O4 | B�� | ���ܲ���ˮ��Һ��Ϊ�������Һ | ||
| C�� | ���ʱ��������ӦΪLi-e-=Li+ | D�� | �ŵ�ʱ��Li+�������ƶ� |
| A�� | ֤��һƿ����ɫ���������������Ƕ�������������ʪ��ĵ⻯��-������ֽ���飬�۲���ֽ��ɫ�ı仯 | |
| B�� | ���ȼ���������ϡ�����ٵμ�KSCN��Һ��δ����Ѫ��ɫ�����ȼ���һ������Fe2O3 | |
| C�� | ����ˮ��pH�����ò�����պȡ��ˮ����pH��ֽ�ϣ������ɫ��ͱ���ɫ���Ƚ� | |
| D�� | ����Cu2+��Fe3+���ӣ����þ���ֽ������������������ֽ��չ������Ũ��ˮѬ�����Լ����Cu2+ |
 �����й��ڷ�֦���˵������ȷ���ǣ�������
�����й��ڷ�֦���˵������ȷ���ǣ�������| A�� | �����к���3�ֺ��������� | |
| B�� | 1 mol��֦��������3mol NaOH�����кͷ�Ӧ | |
| C�� | ��һ�������¿����Ҵ������ᷴӦ���ҷ�Ӧ������ͬ | |
| D�� | ��ʹ������Ȼ�̼��Һ�����Ը��������Һ��ɫ������ɫԭ����ͬ |
| A�� | 150ml 1mol/LNaCl��Һ | B�� | 100ml 3mol/L KClO3��Һ | ||
| C�� | 75ml 2mol/L MgCl2��Һ | D�� | 50ml 1mol/L FeCl3��Һ |
��C2H5OH��g���TC2H5OH��l����H=-Q2kJ/mol
��C2H5OH��g��+3O2��g���T2CO2��g��+3H2O��g����H=-Q3kJ/mol
����˵����ȷ���ǣ�������
| A�� | �ƾ���ȼ���ȡ�H=��Q2-Q3-3Q1��kJ/mol | |
| B�� | �ɢۿ�֪1molC2H5OH��g������������2molCO2��g����3molH2O��g���������� | |
| C�� | H2O��g����H2O��l���ͷų������������Ըù���Ϊ���ȷ�Ӧ | |
| D�� | 23gҺ̬�ƾ���ȫȼ������CO2��g����H2O��l�����ų�������Ϊ��0.5Q2-0.5Q3-1.5Q1��kJ |
