��Ŀ����
13�����������Ҫ�ɷֿɱ�ʾΪFeO•Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��
��֪����Na2CO3+Al2O3$\frac{\underline{\;����\;}}{\;}$2NaAlO2+CO2������Cr2O72-+H2O?2CrO42-+2H+
��������ش��������⣺
��1����������л�ѧ����ʽ����ƽ��
4FeO•Cr2O3+7Na2CO3+8O2 $\frac{\underline{\;����\;}}{\;}$8Na2CrO4+2Fe2O3+8CO2��������CO2�ĽṹʽO=C=O
��2���ữ�����ô��������ҺpH��5����Ŀ����ʹCrO42-ת��Cr2O72-��
��3������Y����Ҫ�ɷ���Al��OH��3��д������Y�����ӷ���ʽAlO2-+CH3COOH+H2O�TAl��OH��3��+CH3COO-��
��4���������ɶಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5������������Ӧ�Ļ�ѧ����ʽΪNa2Cr2O7+2KCl�TK2Cr2O7��+2NaCl��
��6��ij�־ƾ��������У�K2Cr2O7�����������½��Ҵ���������ȩ����������ԭΪ���۸����ӣ��÷�Ӧ�����ӷ���ʽΪCr2O72-+3CH3CH2OH+8H+=2Cr3++3CH3CHO+7H2O��
���� ������ͨ�����գ�����Na2CrO4��Fe2O3��MgO��NaAlO2�Ļ����ϵ��Ȼ���ˮ�ܽ�ù���Fe2O3��MgO����ҺNa2CrO4��NaAlO2���ٵ�����Һ��PH��ʹƫ��������ȫ����������������Һ��PHʹCrO42-ת��ΪCr2O72-�������������Һ�м����Ȼ��أ������ܽ�ȼ�С��K2Cr2O7��
��1�����ݷ�Ӧ�и����ʵ�Ԫ�ػ��ϼ۱仯��֪������+2�۱�Ϊ+3�ۣ�����+3�۱�Ϊ+6�ۣ�����0�۱�Ϊ-2�ۣ����ݻ��ϼ���������Ԫ���غ����ƽ��ѧ����ʽ��������̼������̼��˫����
��2���������ͼ�ͷ�Ӧ�����еõ����ʷ������ữ�����ô��������ҺpH��5Ϊ��ת��CrO42-����ΪCr2O72-��
��3���������̷�����֪���ô������pHֵ������ij���Ϊ�������������ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��4����Һ�еõ����ʾ���ķ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�������ش�
��5��Na2Cr2O7���ܽ��С��K2Cr2O7����Ӧ���ܽ��С�ķ�����У��ݴ���д��ѧ����ʽ��
��6��K2Cr2O7�����������½��Ҵ���������ȩ����������ԭΪ���۸����ӣ�����Ԫ���غ�͵���غ���д���ӷ���ʽ��
��� �⣺������ͨ�����գ�����Na2CrO4��Fe2O3��MgO��NaAlO2�Ļ����ϵ��Ȼ���ˮ�ܽ�ù���Fe2O3��MgO����ҺNa2CrO4��NaAlO2���ٵ�����Һ��PH��ʹƫ��������ȫ����������������Һ��PHʹCrO42-ת��ΪCr2O72-�������������Һ�м����Ȼ��أ������ܽ�ȼ�С��K2Cr2O7��
��1�����ݷ�Ӧ�и����ʵ�Ԫ�ػ��ϼ۱仯��֪������+2�۱�Ϊ+3�ۣ�����+3�۱�Ϊ+6�ۣ�����0�۱�Ϊ-2�ۣ����ݻ��ϼ���������Ԫ���غ����ƽ��ѧ����ʽΪ4FeO•Cr2O3+8Na2CO3+7O2=8Na2CrO4+2Fe2O3+8CO2����������CO2�ĽṹʽΪO=C=O��
�ʴ�Ϊ��4��8��7��8��2��8��O=C=O��
��2���������ͼ�ͷ�Ӧ�����еõ����ʷ������ữ�����ô��������ҺpH��5Ϊ��ת��CrO42-����ΪCr2O72-��
�ʴ�Ϊ��ʹCrO42-ת��Cr2O72-��
��3���������̷�����֪���ô������pHֵ������ij���Ϊ��������������YΪAl��OH��3������Al��OH��3�����ӷ���ʽΪAlO2-+CH3COOH+H2O�TAl��OH��3��+CH3COO-��
�ʴ�Ϊ��Al��OH��3��AlO2-+CH3COOH+H2O�TAl��OH��3��+CH3COO-��
��4�����K2Cr2O7����ķ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ����ȴ�ᾧ��ϴ�ӣ�
��5��Na2Cr2O7���ܽ��С��K2Cr2O7����Ӧ���ܽ��С�ķ�����У����Է�Ӧ�Ļ�ѧ����ʽΪNa2Cr2O7+2KCl�TK2Cr2O7��+2NaCl��
�ʴ�Ϊ��Na2Cr2O7+2KCl�TK2Cr2O7��+2NaCl��
��6��K2Cr2O7�����������½��Ҵ���������ȩ����������ԭΪ���۸����ӣ���Ӧ�����ӷ���ʽΪ Cr2O72-+3CH3CH2OH+8H+=2Cr3++3CH3CHO+7H2O��
�ʴ�Ϊ��Cr2O72-+3CH3CH2OH+8H+=2Cr3++3CH3CHO+7H2O��
���� ���⿼���������Ʊ����̺ͷ����ķ����жϣ��������ʵ�Ӧ�ã������Ϣ�ķ������⣬���������ע������ͻ������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
��1����ͭñ�ܽ�ʱ����H2O2��Ŀ����Cu+H2O2+H2SO4=CuSO4+2H2O���û�ѧ����ʽ��ʾ����
��ͭñ�ܽ���轫��Һ�й���H2O2��ȥ����ȥH2O2�ļ�㷽���Ǽ������У�
��2��Ϊȷ������п�ң���Ҫ�ɷ�ΪZn��ZnO������Ϊ�����������������ʵ������ⶨ��ȥH2O2����Һ��Cu2+�ĺ�����ʵ�����Ϊ��ȷ��ȡһ������ĺ���Cu2+����Һ�ڴ�����ƿ�У�������ˮϡ�ͣ�����pH=3��4���������KI����Na2S2O3����Һ�ζ����յ㣮���������е����ӷ���ʽ���£�
2Cu2++4I-�T2CuI����ɫ����+I2
I2+2S2O32-�T2I-+S4O62-
�ٵζ�ѡ�õ�ָʾ��Ϊ������Һ���ζ��յ�۲쵽������Ϊ��ɫ��ȥ��������ڲ��ָ���
�����ζ�ǰ��Һ��H2O2û�г��������ⶨ��Cu2+�ĺ�������ƫ�ߣ��ƫ�ߡ���ƫ�͡������䡱����
��3����֪pH��11ʱZn��OH��2������NaOH��Һ����[Zn��OH��4]2-���±��г���
�����������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol•L-1���㣩
| ��ʼ������pH | ��ȫ������pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Zn2+ | 5.9 | 8.9 |
�ɳ�ȥͭ����Һ�Ʊ�ZnO��ʵ�鲽������Ϊ��������Һ�м���30%H2O2��ʹ���ַ�Ӧ��
�ڵμ�1.0mol•L-1NaOH��������ҺPHԼΪ5����3.2��pH��5.9����ʹFe3+������ȫ��
�۹��ˣ�
������Һ�еμ�1.0mol•L-1NaOH��������ҺPHԼΪ10����8.9��pH��11����ʹZn2+������ȫ��
�ݹ��ˡ�ϴ�ӡ�����
��900�����գ�
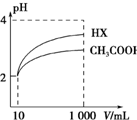 ����HA�ĵ��볣��Ka=$\frac{c��{H}^{+}��•c��{A}^{-}��}{c��HA��}$��25��ʱ����������ĵ��볣�����£�
����HA�ĵ��볣��Ka=$\frac{c��{H}^{+}��•c��{A}^{-}��}{c��HA��}$��25��ʱ����������ĵ��볣�����£�| ���ữѧʽ | HNO2 | CH3COOH | HCN | H2CO3 |
| ���볣�� | 5.1��10-4 | 1.8��10-5 | 6.2��10-10 | K1=4.4��10-7 K2=4.7��10-11 |
�����ʵ���Ũ����ͬ�������ᣬ��pH�ɴ�С��˳����HCN��H2CO3��CH3COOH��HNO2��
�ڷֱ�����������ͬpH��HCl��Һ��CH3COOH��Һ�м���������Zn�ۣ���Ӧ�տ�ʼʱ����H2�����ʣ�v��HCl��=v��CH3COOH�����=����������������ͬ������Ӧ��ȫ������������������m��H2��������m��H2��������
�۽�0.2 mol/L HCN��Һ��0.1 mol/L Na2CO3��Һ�������ϣ�������Ӧ�Ļ�ѧ����ʽΪHCN+Na2CO3�TNaCN+NaHCO3��
��2�������Ϊ10 mL��pH��Ϊ2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1 000 mL��ϡ��������ҺpH�仯��ͼ��ʾ��ϡ�ͺ�HX��Һ��ˮ�����c��H+�� �ȴ�����Һ��ˮ�����c��H+�����볣��Ka��HX��Ka��CH3COOH�������������=����������������ϡ����ͬ��������ǿ����pH�仯�ϴ�ǿ������볣���ϴ�ͼ�п���HX��pH�仯�ϴ�
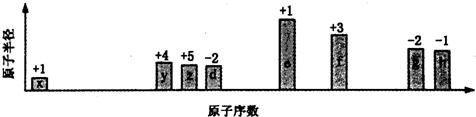
| A�� | ���Ӱ뾶�Ĵ�С˳��e��f��g��h | |
| B�� | ��x�γɼ�����ķе㣺y��z��d | |
| C�� | x��z��d����Ԫ���γɵĻ�������ܺ������Ӽ� | |
| D�� | e��f��g��h����Ԫ�ض�Ӧ����������ˮ�����֮����ܷ�����Ӧ |
| A�� | Na2CO3 | B�� | KMnO4 | C�� | KOH | D�� | H2SO4 |
| A�� | ���շ��������Ƴ�ȼ��������͡����ͣ��ɼ��ỷ����Ⱦ�ͽ�Լ��ʯ��Դ | |
| B�� | ��ѹ�ƵƷ����Ļƹ����Զ��������ǿ��������·�� | |
| C�� | ��ɫ��������ͨ�����������������Լ����ǵĺϽ���Ӧ�÷dz��㷺�Ľ������� | |
| D�� | �ڻ�ҩ���ҹ��Ŵ��Ĵ���֮һ���䷽Ϊ��һ�������ľ̿�������е�����ָ���� |
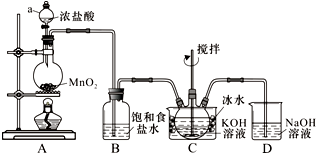 ʵ������ͼ��ʾװ���Ʊ�KClO��Һ������KOH��Fe��NO3��3��Һ��Ӧ�Ʊ���Ч��ˮ��K2FeO4��
ʵ������ͼ��ʾװ���Ʊ�KClO��Һ������KOH��Fe��NO3��3��Һ��Ӧ�Ʊ���Ч��ˮ��K2FeO4��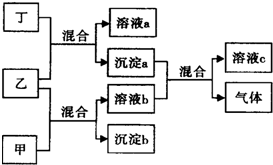 ������ƿ���мס��ҡ���������ǩ����Һ�����ǿ�����K2CO3��Ba��NO3��2��NaHSO4��K2SO4��Һ���ֽ�������ʵ�飬����¼����������ͼ����
������ƿ���мס��ҡ���������ǩ����Һ�����ǿ�����K2CO3��Ba��NO3��2��NaHSO4��K2SO4��Һ���ֽ�������ʵ�飬����¼����������ͼ����