��Ŀ����
�����й����������漰�IJ�����ԭ��˵����ȷ���ǣ�������
| A���ϳɰ����������н�NH3Һ�����룬�ɼӿ�����Ӧ���ʣ����N2��H2��ת���� |
| B��������п����п���Դ������������ƹ�����Ҫ���Ƶ���ǿ�ȡ���ҺpH������ |
| C��������ǰ���п���Dz��������������������������Ӷ�����������ʴ���� |
| D����⾫��ͭʱ�������ܽ�ͭ������������������ͭ����������� |
���㣺��ҵ�ϳɰ�,�����ĵ绯ѧ��ʴ�����,���ԭ��
ר�⣺��ѧӦ��
������A����С�������Ũ�ȣ�����ʹ��ѧ��Ӧ���ʼ�����
B����Ƴ��У��Ƽ����������Ʋ���������������Һ�Ǻ��жƲ���������ӵ�����Һ�ݴ˻ش�
C���������Ľ������Բ�������������������������
D����⾫��ͭʱ�������Ǵ�ͭ�����к��н���Cu������Zn��Fe�Ȼ��ý����������Ǿ�ͭ��
B����Ƴ��У��Ƽ����������Ʋ���������������Һ�Ǻ��жƲ���������ӵ�����Һ�ݴ˻ش�
C���������Ľ������Բ�������������������������
D����⾫��ͭʱ�������Ǵ�ͭ�����к��н���Cu������Zn��Fe�Ȼ��ý����������Ǿ�ͭ��
���
�⣺A���ϳɰ����������н�NH3Һ�����룬����С�����ﰱ����Ũ�ȣ�����ʹ��ѧ��Ӧ���ʼ��������������N2��H2��ת���ʣ���A����
B��������п����п���Դ������������������������ƹ�����Ҫ���Ƶ���ǿ�ȡ���ҺpH����������B����
C����ԭ��ظ����Ľ������ٱ���ʴ����ԭ��������Ľ�����������������ǰ���п�壬п�����ͺ�ˮ����ԭ��أ�п�������������������������ñ�������Ϊ������������������������C��ȷ��
D����⾫��ͭʱ�������Ǵ�ͭ������Zn��Fe�Ȼ��ý������ڽ���ͭʧ���ӣ�������ͭ���ӵĵ��ӵĹ��̣�ͬһʱ���ڣ���ת�Ƶ���һ��������£������ܽ�ͭ����������������ͭ������С����D����
��ѡC��
B��������п����п���Դ������������������������ƹ�����Ҫ���Ƶ���ǿ�ȡ���ҺpH����������B����
C����ԭ��ظ����Ľ������ٱ���ʴ����ԭ��������Ľ�����������������ǰ���п�壬п�����ͺ�ˮ����ԭ��أ�п�������������������������ñ�������Ϊ������������������������C��ȷ��
D����⾫��ͭʱ�������Ǵ�ͭ������Zn��Fe�Ȼ��ý������ڽ���ͭʧ���ӣ�������ͭ���ӵĵ��ӵĹ��̣�ͬһʱ���ڣ���ת�Ƶ���һ��������£������ܽ�ͭ����������������ͭ������С����D����
��ѡC��
���������⿼��ѧ����ѧ��Ӧ���ʺͻ�ѧƽ���ƶ�����ҵ��������Ĺ�ҵԭ�������صĹ���ԭ���Լ���⾫��֪ͭʶ�������ۺ�֪ʶ�Ŀ��飬�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�Ƚ�MnO2��CuO��H2O2�ֽⷴӦ�Ĵ�������С��ʵ���У���������������������0.1g��3%��H2O2��Һ��ȡ5mL����ѡ���ʵ��װ���ǣ�������
A��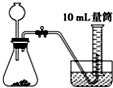 |
B��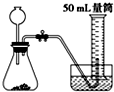 |
C��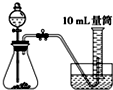 |
D��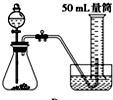 |
���������в���ȷ���ǣ�������
| A����ˮ��ʯ�������ܵ�ԭ�����ܽ���CO2����ˮ��CaCO3���������˿�����Ca��HCO3��2��Ե�� |
| B��25��ʱ��������XY��AB3��Ksp�ֱ�Ϊ1.0��10-10��2.7��10-15������¶��£����ߵı�����Һ��c��X+��һ������c��A3+�� |
| C��25��ʱ��pH=ll��KaA��Һ��pH=11��KOH��Һ��ˮ�����c��OH-��ǰ���Ǻ��ߵ�108�� |
| D�����������Լ��ܰ�NaCl��AlCl3��Ba��OH��2������Һ������� |
�����и�����Һ�У�����һ���ܴ���������ǣ�������
| A������0.1 mol?L-1H+����Һ�У�NO3-��Na+��Cl-��Fe2+ |
| B����ɫ������Һ�У�K+��Mg2+��Cl-��SO42- |
| C��ǿ������Һ�У�Na+��SO42-��HCO3-��K+ |
| D����������Ӧ����H2����Һ�У�Na+��Al3+��Cl-��SO42- |


 ��
��
 ��ʾ��ͬ��һ��ԭ�ӣ�������֮������ߴ�����ѧ�����絥����˫���ȣ���
��ʾ��ͬ��һ��ԭ�ӣ�������֮������ߴ�����ѧ�����絥����˫���ȣ���
