��Ŀ����
9�������Na��Mg��Si��S��Br����Ԫ�صĵ��ʵķе㣬����b��e�����Ⱥ͵�������壮| ���� | a | b | c | d | e |
| �е㣨�棩 | 58.8 | 882.9 | 444.7 | 2��355 | 1��107 |
��2��д��d��Ԫ��ԭ�ӵ����������Ų�ʽ3s23p2����Ԫ��ԭ�Ӻ����й���5��������ͬ�ĵ��ӣ�
��3��д��c����̬�⻯���ˮ��Һ��b��Ԫ�ص�����������Ӧˮ���ﷴӦ�����ӷ���ʽH2S+2OH-=S 2-+H2O��
���� �������Ⱥ͵�������壬b��e���Ⱥ͵�������壬b��e�ǽ��������������۷е���������йأ���������ԭ�Ӱ뾶�ɷ��ȣ�b�۵�С��e����b��Na��e��Mg��
ԭ�Ӿ����۷е�ϸߣ�����d��Si��
�����£�S�ǹ��塢����Һ�壬S���۷е�����壬��a���塢c����
��1��a���壬�����غ�ɫ����λ�ڵ������ڵ�VIIA�壻
��2��d��SiԪ�أ�d��Ԫ��ԭ�ӵ������ΪM�㣬�����������2��3s���ӡ�2��3p���ӣ�
��Ԫ��ԭ�Ӻ������м����ܼ����м���������ͬ�ĵ��ӣ�
��3��c��S��c����̬�⻯���ˮ��ҺΪ�����ᣬb��Ԫ�ص�����������Ӧˮ������NaOH�����߷����кͷ�Ӧ��
��� �⣺�������Ⱥ͵�������壬b��e���Ⱥ͵�������壬b��e�ǽ��������������۷е���������йأ���������ԭ�Ӱ뾶�ɷ��ȣ�b�۵�С��e����b��Na��e��Mg��
ԭ�Ӿ����۷е�ϸߣ�����d��Si��
�����£�S�ǹ��塢����Һ�壬S���۷е�����壬��a���塢c����
��1��a���壬�����غ�ɫ����ԭ�Ӻ�����4�����Ӳ㡢�������7�����ӣ�λ�ڵ������ڵ�VIIA�壬
�ʴ�Ϊ������أ��������ڵ�VIIA�壻
��2��d��SiԪ�أ�d��Ԫ��ԭ�ӵ������ΪM�㣬�����������2��3s���ӡ�2��3p���ӣ������������Ų�ʽΪ3s23p2��
��Ԫ��ԭ�Ӻ������м����ܼ����м���������ͬ�ĵ��ӣ���ԭ�Ӻ�����5���ܼ�������5�ֲ�ͬ�����ĵ��ӣ�
�ʴ�Ϊ��3s23p2��5��
��3��c��S��c����̬�⻯���ˮ��ҺΪ�����ᣬb��Ԫ�ص�����������Ӧˮ������NaOH�����߷����кͷ�Ӧ�������ƺ�ˮ�����ӷ���ʽΪH2S+2OH-=S 2-+H2O���ʴ�Ϊ��H2S+2OH-=S 2-+H2O��
���� ���⿼��ԭ�ӽṹ��Ԫ�����ʣ�Ϊ��Ƶ���㣬�漰���ӷ���ʽ����д��ԭ�Ӻ�������Ų���ԭ�ӽṹ��֪ʶ�㣬��ȷԭ�ӽṹ��Ԫ�ػ����������ǽⱾ��ؼ�����Ŀ�ѶȲ���

| A�� | �� | B�� | �� | C�� | ��ԭ�� | D�� | ������ |
| A�� | FeO | B�� | Fe2O3 | C�� | Fe3O4 | D�� | FeO��Fe2O3 |
��HCN?H++CN-��H1��0 K1
��CN-+H2O?HCN+OH-��H2��K2
�����£�K1=6.2��10-10������������������ʵ���Ũ�ȵ�HCN��NaCN��Һ��ϣ�����������ȷ���ǣ�������
| A�� | K2��1.6��10-3 | B�� | 2c��Na+��=c��HCN��+c��CN-�� | ||
| C�� | �����Һ��pH��7 | D�� | �Ի����Һ���£�K1����K2��С |
| A�� | Ǧ�����ڳ������У������������������� | |
| B�� | ��Ӧ2Mg��s��+CO2��g��=C��s��+2MgO��s�����Է����У���÷�Ӧ�ġ�H��0 | |
| C�� | NH3•H2O��Һ��ˮϡ�ͺ���Һ��$\frac{c��N{H}_{3}•{H}_{2}O��}{c��N{{H}_{4}}^{+}��}$��ֵ��С | |
| D�� | �����£�pH��Ϊ5���������Ȼ����Һ�У�ˮ�ĵ���̶���ͬ |

 ��
�� ����I��G �Ļ�ѧ����ʽ
����I��G �Ļ�ѧ����ʽ ��
��

 ��
�� ����дһ�֣��������������칹��
����дһ�֣��������������칹��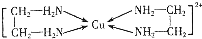 �������ڽ���Cr��Fe��Cu�ڿ�ѧ�о���ҵ�����ж�����Ҫ����;����ش��������⣺
�������ڽ���Cr��Fe��Cu�ڿ�ѧ�о���ҵ�����ж�����Ҫ����;����ش��������⣺