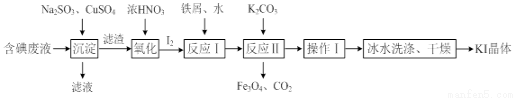
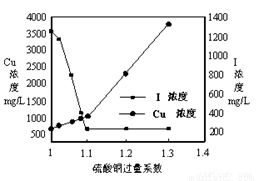
��Ŀ����
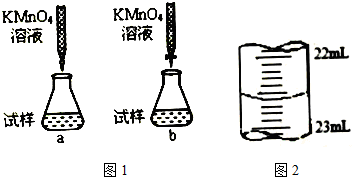
14��ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ������Ũ��Ϊ0.1mol/L�ı�NaOH��Һ���еζ����ظ������ζ�����2�Σ���¼�������£�| ʵ���� | NaOH��Һ�����mL�� | ��������������mL�� |
| 1 | 22.62 | 20.00 |
| 2 | 22.72 | 20.00 |
| 3 | 22.80 | 20.00 |
��2�������������ݣ��ɼ�����������Ũ��ԼΪ0.11mol/L��������λ��Ч���֣���
��3��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���DF������ѡ�۷֣�
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D������ǰNaOH�����л���Na2CO3����
E��NaOH����Һ����������е�CO2��Ӧ������Na2CO3
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��
���� ��1������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻����ָʾ���ı�ɫ��Χȷ��PH��
��2�����ж����ݵĺ����ԣ������NaOH��Һ�����Ȼ������c�����⣩=$\frac{c��������V������}{V�����⣩}$������ɣ�
��3������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1��NaOH��Һ�ζ����ᣬ��̪��ָʾ���������һ��NaOH��Һ����ʱ����Һ����ɫǡ�ñ��dz��ɫ���Ұ�����ڲ���ɫΪ�ζ��յ㣬��̪�ı�ɫ��Χ��8.2��10�����Եζ��յ�����ҺpHΪ8.2��10��
�ʴ�Ϊ�����һ������������Һ���룬��Һ����ɫǡ�ñ��dz��ɫ���Ұ�����ڲ���ɫ��8.2��10��
��2��ʵ��1���ϴ���ȥ���ó�V������=$\frac{22.72+22.80}{2}$=22.76mL������c�����⣩=$\frac{c��������V������}{V�����⣩}$=$\frac{0.1mol/L��22.76mL}{20.00mL}$=0.11mol/L���ʴ�Ϊ��0.11mol/L��
��3��A���ζ��յ����ʱ���Ӷ������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫС����A����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ������Һ��ϡ�ͣ������ʵ���ƫС�����V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫС����B����
C����ƿˮϴ��δ�������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩���䣬��C����
D��1molNaOH��40g����1molHCl��1molNa2CO3��106g����2molHCl�����������NaOH��Na2CO3�����ᷴӦ��NaOH���ĵ�����࣬����������ᷴӦʱ����Ҫ�Ļ���Na2CO3��NaOH����Һ�϶࣬����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ��D��ȷ��
E��NaOH����Һ����������е�CO2��Ӧ������Na2CO3���������غ㣬��V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩���䣬��E����
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ��F��ȷ��
��ѡDF��
���� ���⿼��������к͵ζ�ʵ�鼰�������������к͵ζ��IJ�����������ɷ����ķ����ǽ���ؼ���ע�����ݹ�ʽc�����⣩=$\frac{c��������V������}{V�����⣩}$��������������Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ������ˮ�����ζ��ܺ�װ���������еζ� | |
| B�� | ������ˮ������ƿ������NaOHҺ��ϴ������װ��һ�������NaOH��Һ | |
| C�� | �ü�ʽ�ζ���ȡ10.00mLNaOH��Һ����������ˮϴ������ƿ�У��ټ�����������ˮ���еζ� | |
| D�� | ������Һ��ȡ10.00mLNaOH��Һ��������ƿ����Һ�ܼ��촦Һ�崵�� |
| A�� | ������ӦҲ���ڼӳɷ�Ӧ | |
| B�� | ������Ӧ������ȥ�ǻ�������ȥ��ԭ�� | |
| C�� | Ũ������������Ӧ��ֻ����������� | |
| D�� | ���������Ľṹ��ʽΪCH3COOCH3 |
| ѡ�� | ���� | ʵ������ | ���� |
| A | ����ȡ����ȡ���CCl4��Һ�еĵ� | ���CCl4��Һ�м�������ˮ�������á���Һ | ����ˮ���ܽ�ȱ���CCl4�д� |
| B | ��FeCl3��Һ�м�������Fe�۳�ַ�Ӧ���ٵμӼ��λ�ɫ���軯����Һ | ������ɫ���� | 2Fe3++Fe�T3Fe2+ 3Fe2++2[Fe��CN��6]3-�TFe3[Fe��CN��6]2�� |
| C | ��0.1mol/L��Fe��NO3��2��Һ�еμ����� | ���Թ���ɫ���� | H+����Fe2+ˮ�� |
| D | �õ����ʴ�ˮ��Һ������ | ��CuSO4��Һ���뵰�����з������� | �ؽ���������Һ�ɽ��͵����ʵ��ܽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
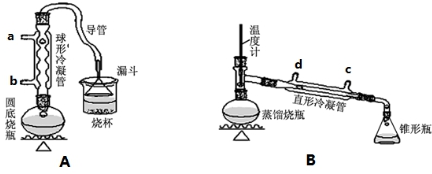
��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���Ǽȿ������ճ�֣��ֿ��Է�ֹ����������װ���ж��õ��������ܣ�Aװ������ˮ��b������ĸ���ţ����룬Bװ������ˮ��c������ĸ���ţ����룮
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ����ab��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С�-CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���𣺲�����������1-�嶡��Ҳ����-CH2CH2CH2CH3��
��4��Ϊ�˽�һ���ᴿ1-�嶡�飬��С��ͬѧ�������л�����й��������±���
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |