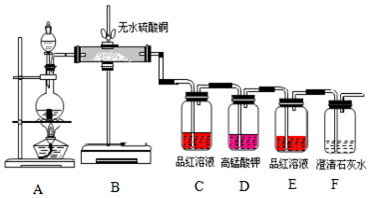
��Ŀ����
12�� ijʵ��С����0.50mol•L-1NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol•L-1NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��������0.50mol•L-1NaOH��Һ
��1����ʵ���д�ԼҪʹ��245mLNaOH��Һ��������Ҫ����NaOH����5.0g��
��2����ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ����abe��
| ���� | ������ƽ �������룩 | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
��1��������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ��Ǽ���ʵ������е�������ʧ�����ձ��粻��Ӳֽ�壬��õ��к�����ֵ��ƫС���ƫ����ƫС������Ӱ�족��������ճ�����ʵ�ʸ�ʵ���ڱ��±��У����ò�Ʒ��Ч�����ã�
��3��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3kJ•mol-1��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol����
��4��ȡ50mLNaOH��Һ��30mL������Һ����ʵ�飬ʵ�����������
| ������� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.6 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڽ�����Ϊ0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ���ܶȶ���1g•cm-3���кͺ�������Һ�ı�����c=4.18J•��g•�棩-1�����к��ȡ�H=-53.5kJ/mol��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ��acd��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��ʵ���и���60mL0.5mol/L�����50mL0.55mol•L-1�������ƽ��з�Ӧ��������ʵ����ȣ����ų�����������ȣ�����Ȼ���ȣ���ͬ����������к�����ȼ��������к�����ָ�������кͷ�Ӧ����1molˮ���ų�������Ϊ���ģ������ᡢ��������أ���
���� ��1�����ݹ�ʽm=nM=cVM�������������Ƶ�����������û��245mL������ƿ��
��2����������Ҫ�ڲ��������г��������ݳ������������������õ��������ش�
��1���������ȿ죬������ʧ�ࣻ
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹��������ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ���ճ����������Ǿ����õ����±����ڱ��±��н���ʵ�鱣��Ч������ã�
��3��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������Ӧ����1molҺ̬ˮ��
��4�����ȼ����ÿ����������ⶨ���¶ȲȻ���������ϴ�����ݣ���������¶Ȳ�ƽ��ֵ��
�ڸ���Q=m•c•��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��a��ʵ��װ�ñ��¡�����Ч�������ɢʧ�ϴ�
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ��
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ��У�����ɢʧ�ϴ�
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ��������ʼ�¶�ƫ�ߣ�
�ܷ�Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��� �⣺��1������ƿû��245mL���ģ�ֻ����250mL���ģ���Ҫ����NaOH����m=nM=cVM=0.5mol/L��0.25L��40g/mol=5.0g��
�ʴ�Ϊ��5.0��
��2����������Ҫ��С�ձ��г������������������������õ���������ƽ���ձ���ҩ�ף�
�ʴ�Ϊ��abe��
��1�����ܽ����β����������Ϊͭ˿���������Ϊͭ˿��������ȵ������壻
�ʴ�Ϊ��Cu���ȿ죬������ʧ��
��2�����кͷ�Ӧ�У�����ȷ��������ɢʧ��Ӧ���װ�õı���Ч�������ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С�����ճ�����ʵ�ʸ�ʵ���ڱ��±���Ч�����ã�
�ʴ�Ϊ�����װ�õı���Ч����ƫС�����±���
��3��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������Ӧ����1molҺ̬ˮ���Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2���ٵ�һ�βⶨ�¶Ȳ�Ϊ3.5�棬�ڶ��βⶨ���¶Ȳ�Ϊ4.0�棬�����βⶨ���¶Ȳ�Ϊ3.9�棬���Ĵβⶨ���¶Ȳ�Ϊ4.1�棬ʵ��1�����̫��Ҫ��ȥ�������¶Ȳ��ƽ��ֵΪ4.0�棬
�ʴ�Ϊ��4.0��
��50mL 0.50mol•L-1����������30mL0.50mol•L-1������Һ�����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80mL��1g/cm3=100g���¶ȱ仯��ֵΪ��T=4.0�棬������0.025molˮ�ų�������Ϊ��Q=m•c•��T=80g��4.18J/��g•�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-$\frac{1.3376kJ}{0.025mol}$=-53.5kJ/mol
�ʴ�Ϊ��-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b����
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���c��ȷ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��acd��
�ܷ�Ӧ�ų����������������Լ�������Ķ����йأ�ʵ���и���60mL0.5mol/L�����50mL0.55mol•L-1�������ƽ��з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ��к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ��к�����ָ�������кͷ�Ӧ����1molˮ���ų�������Ϊ���ģ������ᡢ��������أ�
���� ������Ҫ�����к��ȵIJⶨ����Ŀ�Ѷ��еȣ��漰��Һ�����ơ��Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�������Լ��ⶨ��Ӧ�ȵ��������⣬����������ѧ���ķ�����������ѧʵ��������

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�| A�� | ZnΪ��ص����� | |
| B�� | ������ӦʽΪ2FeO42-+10H++6e-�TFe2O3+5H2O | |
| C�� | �õ��ʹ���겻����㶪����Ӧ������� | |
| D�� | ��ع���ʱOH-��Ǩ�� |
| A�� | ��ճ����ԭ�� 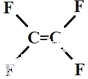 Ϊ������� Ϊ������� | |
| B�� | ����������1�������ɸ���CH2��ԭ���ŵ��л������Ϊͬϵ�� | |
| C�� | ʯ�ͷ����������仯��ú��������Һ���ǻ�ѧ�仯 | |
| D�� | ����ϩ���ϴ����ж���������װʳƷ |
| A�� | ̼���裨SiC�� | B�� | ���ᣨCH3COOH�� | C�� | ��ϩ��C2H4�� | D�� | �ƾ� |
| A�� | 5.3g | B�� | 10.6g | C�� | 14.3g | D�� | 16.4g |
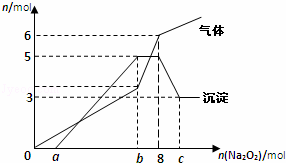 ��Na2O2���뵽����H+��Mg2+��Al3+��NH4+�Ļ��Һ�в��ȣ�������������������ʵ�����mol��������Na2O2���ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����ԭ��Һ�е�Mg2+��Al3+��NH4+�����ʵ����ֱ�Ϊ��������
��Na2O2���뵽����H+��Mg2+��Al3+��NH4+�Ļ��Һ�в��ȣ�������������������ʵ�����mol��������Na2O2���ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����ԭ��Һ�е�Mg2+��Al3+��NH4+�����ʵ����ֱ�Ϊ��������| A�� | 2mol��3mol��6mol | B�� | 3mol��2mol��6mol | C�� | 2mol��3mol��4mol | D�� | 3mol��2mol��2mol |
 ������Ӫ���ḻ���������ʡ����ۡ���֬��Ҷ�ᡢά����C��Ҷ���أ�Ӫ���ɷ�λ��ͬ���߲�֮�ף�����Ϊ���߲˻ʹڡ���
������Ӫ���ḻ���������ʡ����ۡ���֬��Ҷ�ᡢά����C��Ҷ���أ�Ӫ���ɷ�λ��ͬ���߲�֮�ף�����Ϊ���߲˻ʹڡ���  X��Y���ֹ�����ܽ������ͼ��
X��Y���ֹ�����ܽ������ͼ��