��Ŀ����
20��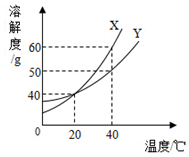 X��Y���ֹ�����ܽ������ͼ��
X��Y���ֹ�����ܽ������ͼ����1��X��Y�������ʵ��ܽ�ȱ仯���ƵĹ�ͬ�����ܽ�ȶ����¶ȵ����߶�����
��2���¶�Խ�ߣ�x�ı�����Һ��X����A����Խ���Խ��ԽС������
��3����a1gY�ı�����Һ���½ᾧ���������壨�����ᾧˮ��mg�õ���Һa2 g����a1��a2��m�Ĺ�ϵ��b������ţ���
a�� a1��a2+m b��a1=a2+m
c�� a1��a2+m d����ȷ��
��4�������¶Ȳ��䣬X�ı�����Һ��Y�ı�����Һ��ϣ���X��Y����Ӧ����õ�����Һ��d
a��X�ı�����Һ��Y�ı�����Һ b�� X�IJ�������Һ��Y�ı�����Һ
c�� X�ı�����Һ��Y�IJ�������Һ d�� X�IJ�������Һ��Y�IJ�������Һ��
���� ��1��ͼ��������Ϊ�ܽ�ȣ�����Ϊ�¶ȣ��¶�Խ�ߣ��ܽ��Խ������Ŀ��Ϣ���ܽ�����߿�֪��X��Y���ֹ������ʵ��ܽ�ȣ��������¶����߶�����X�仯��һЩ��Y�仯СһЩ��
��2���¶�Խ�������ܽ��Խ����Һ���ܽ�����Խ�࣬�¶�Խ�ߣ�X�ı�����Һ��X����������Խ����Ϊ�¶�Խ�ߣ�100gˮ���ܽ������Խ�ࣻ
��3����a1gY�ı�����Һ���½ᾧ���������壨�����ᾧˮ��mg���õ���Һa2g����a1��a2��m�Ĺ�ϵ�ǣ�a1=a2+m��
��4�������¶Ȳ��䣬X�ı�����Һ��Y�ı�����Һ��ϣ�X��Y����Ӧ����õ�����Һ�ǣ�X�IJ�������Һ��Y�IJ�������Һ����ΪX��Y������û�б仯��������Һ�������ˣ�
��� �⣺��1�����ܽ�����߿�֪��X��Y���ֹ������ʵ��ܽ�ȣ��������¶����߶�����
�ʴ�Ϊ���ܽ�ȶ����¶����߶�����
��2���¶�Խ�ߣ�X�ı�����Һ��X����������Խ����Ϊ�¶�Խ�ߣ�100gˮ���ܽ������Խ�࣬
�ʴ�Ϊ��Խ��
��3����a1gY�ı�����Һ���½ᾧ���������壨�����ᾧˮ��mg���õ���Һa2g����a1��a2��m�Ĺ�ϵ�ǣ�a1=a2+m���ʴ�Ϊ��b��
��4�������¶Ȳ��䣬X�ı�����Һ��Y�ı�����Һ��ϣ�x��Y����Ӧ����õ�����Һ�ǣ�X�IJ�������Һ��Y�IJ�������Һ����ΪX��Y������û�б仯��������Һ�������ˣ�
�ʴ�Ϊ��d��
���� ���⿼���ܽ�����ߵ����弰Ӧ�ã������ܽ�����߿��жϱ�����Һ�벻������Һת���ķ����������ܽ�����¶����߶���������ʣ��ɲ�������Һ��ɱ�����Һֻ���ǽ��£���Ŀ�Ѷ��еȣ�

| A | B | C | D | |
| ��Ʒ |  |  |   |  |
| ��Ч�ɷ� | NaCl | Na2CO3 | Al��OH��3 | Ca��ClO��2 |
| ��; | ����ζƷ | �����ͷ� | ������ҩ | �������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
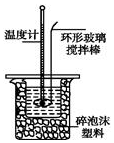 ijʵ��С����0.50mol•L-1NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol•L-1NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��������0.50mol•L-1NaOH��Һ
��1����ʵ���д�ԼҪʹ��245mLNaOH��Һ��������Ҫ����NaOH����5.0g��
��2����ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ����abe��
| ���� | ������ƽ �������룩 | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
��1��������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ��Ǽ���ʵ������е�������ʧ�����ձ��粻��Ӳֽ�壬��õ��к�����ֵ��ƫС���ƫ����ƫС������Ӱ�족��������ճ�����ʵ�ʸ�ʵ���ڱ��±��У����ò�Ʒ��Ч�����ã�
��3��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3kJ•mol-1��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol����
��4��ȡ50mLNaOH��Һ��30mL������Һ����ʵ�飬ʵ�����������
| ������� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.6 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڽ�����Ϊ0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ���ܶȶ���1g•cm-3���кͺ�������Һ�ı�����c=4.18J•��g•�棩-1�����к��ȡ�H=-53.5kJ/mol��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ��acd��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��ʵ���и���60mL0.5mol/L�����50mL0.55mol•L-1�������ƽ��з�Ӧ��������ʵ����ȣ����ų�����������ȣ�����Ȼ���ȣ���ͬ����������к�����ȼ��������к�����ָ�������кͷ�Ӧ����1molˮ���ų�������Ϊ���ģ������ᡢ��������أ���
 ijͬѧ�ڰ���������������5������ ����ͼ��ʾ���������������ʼ䷢���ķ�Ӧ�У�û���漰�Ļ�����Ӧ�����ǣ�������
ijͬѧ�ڰ���������������5������ ����ͼ��ʾ���������������ʼ䷢���ķ�Ӧ�У�û���漰�Ļ�����Ӧ�����ǣ�������| A�� | �ֽⷴӦ | B�� | ���ֽⷴӦ | C�� | ���Ϸ�Ӧ | D�� | �û���Ӧ |




 ά������������Ҫ��Ӫ�����ʣ�ͼΪijƷ��ά����C����Ƭ˵����IJ������ݣ�������Ƭ�����ӵ���ɫ��������ƣ���ζ�����Ǿ��ƣ�����Ƭ����ˮʱ���������ڡ�Ч�������������ӵľ�ʯ���̼�����Ʒ�Ӧ�ͷų������壮
ά������������Ҫ��Ӫ�����ʣ�ͼΪijƷ��ά����C����Ƭ˵����IJ������ݣ�������Ƭ�����ӵ���ɫ��������ƣ���ζ�����Ǿ��ƣ�����Ƭ����ˮʱ���������ڡ�Ч�������������ӵľ�ʯ���̼�����Ʒ�Ӧ�ͷų������壮 ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�
ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�