��Ŀ����
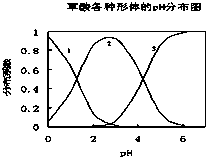 ���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ����1��ͼ������1��ʾ
��2��Na2C2O4��Һ�У�
| c(Na+) |
| c(C2O42-) |
| c(Na+) | ||
c(C2
|
��3����֪NaHC2O4��Һ�����ԣ������£���10mL 0.1mol/L H2C2O4��Һ�еμ�0.1mol/L NaOH��Һ������NaOH��Һ��������ӣ�����Һ��c��Na+��=2c��C2O42-��+c��HC2O4-��ʱ����Һ��
��4���������ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��������Һ��
a��Na2C2O4 b��NaHC2O4c��H2C2O4d����NH4��2C2O4e��NH4HC2O4
�����й���������Һ��˵���У���ȷ����
A����Һb�У�c��C2O42-����c��H2C2O4��
B����Һb�У�c��H2C2O4��+c��OH-��=2c��C2O42-��+c��H+��
C����Һd��e�ж�����c��NH4+��+c��H+��=c��HC2O4-��+2c��C2O42-��+c��OH-��
D��������Һ������c��H2C2O4��+c��HC2O4-��+c��C2O42-��=0.1mol?L-1
��������Һ��c��H2C2O4���ɴ�С���е�˳����
���㣺����Ũ�ȴ�С�ıȽ�,���������ˮ��Һ�еĵ���ƽ��,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
��������1��pHԽС��H2C2O4��Ũ��Խ��
��2���������еIJ�������������������ӣ���ˮ�⣬C2O42-��Ca2+�γɳ�����
��3�����ݵ���غ�ȷ����Һ�������Ӻ�����������Ũ����Դ�С���Ӷ�ȷ����Һ����ԣ�����������Һ�����ԣ�Ҫʹ��Һ�����ԣ�����������Ӧ����������
��4����A��NaHC2O4��Һ�����ԣ�˵������������ӵ���̶ȴ���ˮ��̶ȣ�
B����Һb�У����ڵ���غ�������غ㣻
C���κ���Һ�ж����ڵ���غ㣻
D���κ���Һ�ж����������غ㣻
�ڲ��������ˮ��̶ȴ��ڲ���������ӣ�������������ʣ�����̶Ƚ�С��
��2���������еIJ�������������������ӣ���ˮ�⣬C2O42-��Ca2+�γɳ�����
��3�����ݵ���غ�ȷ����Һ�������Ӻ�����������Ũ����Դ�С���Ӷ�ȷ����Һ����ԣ�����������Һ�����ԣ�Ҫʹ��Һ�����ԣ�����������Ӧ����������
��4����A��NaHC2O4��Һ�����ԣ�˵������������ӵ���̶ȴ���ˮ��̶ȣ�
B����Һb�У����ڵ���غ�������غ㣻
C���κ���Һ�ж����ڵ���غ㣻
D���κ���Һ�ж����������غ㣻
�ڲ��������ˮ��̶ȴ��ڲ���������ӣ�������������ʣ�����̶Ƚ�С��
���
�⣺��1������ͼ��֪����Һ��pHԽС�������Ũ��Խ����������1Ϊ���ᣬ�ʴ�Ϊ��H2C2O4��
��2���������еIJ�������������������ӣ���ˮ�⣬���Բ�������Һ�У�
��2���ò�������Һ�е����Ȼ�����Һ��C2O42-��Ca2+�γɳ���������C2O42-Ũ�Ƚ��ͣ�
���ӣ����ӷ���ʽΪC2O42-+Ca2+=CaC2O4�����ʴ�Ϊ������C2O42-+Ca2+=CaC2O4����
��3�����ݵ���غ��c��Na+��+c��H+��=2c��C2O42-��+c��HC2O4-��+c��OH-����c��Na+��=2c��C2O42-��+c��HC2O4-������c��H+��=c��OH-����������Һ�����ԣ�
��������Һ�����ԣ�Ҫʹ��Һ�����ԣ�����������Ӧ��������������V��NaOH����10mL��
�ʴ�Ϊ���У�����
��4����A��NaHC2O4��Һ�����ԣ�˵������������ӵ���̶ȴ���ˮ��̶ȣ���c��C2O42-����c��H2C2O4�����ʴ���
B����Һb�У����ڵ���غ�������غ㣬���������غ��c��Na+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-�������ݵ���غ��c��H+��+c��Na+��=c��HC2O4-��+2c��C2O42-��+c��OH-�������Ե�c��H+��+c��H2C2O4��=2c��C2O42-��+c��OH-�����ʴ���
C���κ���Һ�ж����ڵ���غ�c��NH4+��+c��H+��=c��HC2O4-��+2c��C2O42-��+c��OH-��������ȷ��
D���κ���Һ�ж����������غ㣬���������غ��c��H2C2O4��+c��HC2O4-��+c��C2O42-��=0.1mol?L-1������ȷ��
��ѡCD��
�ڲ��������ˮ��̶ȴ��ڲ���������ӡ�笠����Ӵٽ���������ӻ�����������ˮ�⣬�������̶Ƚ�С��������Һ��c��H2C2O4���ɴ�С���е�˳����c��e��b��d��a���ʴ�Ϊ��c��e��b��d��a��
��2���������еIJ�������������������ӣ���ˮ�⣬���Բ�������Һ�У�
| c(Na+) |
| c(C2O42-) |
| c(Na+) |
| c(C2O42-) |
��3�����ݵ���غ��c��Na+��+c��H+��=2c��C2O42-��+c��HC2O4-��+c��OH-����c��Na+��=2c��C2O42-��+c��HC2O4-������c��H+��=c��OH-����������Һ�����ԣ�
��������Һ�����ԣ�Ҫʹ��Һ�����ԣ�����������Ӧ��������������V��NaOH����10mL��
�ʴ�Ϊ���У�����
��4����A��NaHC2O4��Һ�����ԣ�˵������������ӵ���̶ȴ���ˮ��̶ȣ���c��C2O42-����c��H2C2O4�����ʴ���
B����Һb�У����ڵ���غ�������غ㣬���������غ��c��Na+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-�������ݵ���غ��c��H+��+c��Na+��=c��HC2O4-��+2c��C2O42-��+c��OH-�������Ե�c��H+��+c��H2C2O4��=2c��C2O42-��+c��OH-�����ʴ���
C���κ���Һ�ж����ڵ���غ�c��NH4+��+c��H+��=c��HC2O4-��+2c��C2O42-��+c��OH-��������ȷ��
D���κ���Һ�ж����������غ㣬���������غ��c��H2C2O4��+c��HC2O4-��+c��C2O42-��=0.1mol?L-1������ȷ��
��ѡCD��
�ڲ��������ˮ��̶ȴ��ڲ���������ӡ�笠����Ӵٽ���������ӻ�����������ˮ�⣬�������̶Ƚ�С��������Һ��c��H2C2O4���ɴ�С���е�˳����c��e��b��d��a���ʴ�Ϊ��c��e��b��d��a��
���������⿼��������Ũ�ȴ�С�Ƚϣ���ȷ��Һ�е����ʼ��������ǽⱾ��ؼ������ݵ���غ㡢�����غ������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
���и�����������ѧ��Ӧ������ͬһ��Ӧ���͵�һ���ǣ�������
| A���ɱ������������ɱ��ƻ����� |
| B������ϩ��1��2-�������飻��������һ������ |
| C����ϩʹ��ˮ��ɫ����ϩʹ���Ը��������Һ��ɫ |
| D���ɱ����屽�����Ҵ����廯�ⷴӦ |
�������ӷ���ʽ��д��ȷ���ǣ�������
| A��ˮ����ˮ����ʳ�׳�ȥ��2H++CaCO3=Ca2++CO2��+H2O 2H++Mg��OH��2=Mg2++2H2O |
| B����NaHSO4��Һ�е���Ba��OH��2��Һ������H++SO42-+Ba2++OH-=BaSO4��+H2O |
| C����FeI2��Һ��ͨ������Cl2 2I-+Cl2=I2+2Cl- |
| D���������Ũ�ȵ�Ba��OH��2ϡ��Һ��NH4HCO3ϡ��Һ��ϣ�Ba2++2OH-+NH4++HCO3-=BaCO3��+NH3��+2H2O |