��Ŀ����
���г����µ�������Һ��
��0.01molgL-1CH3COOH��Һ����0.01mo1-1HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01mol-1CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01mol-1HCl��Һ��pH=12��NaOH��Һ��������������Һ��
��1������ˮ�ĵ���̶������� ������ţ���ͬ����ˮ�ĵ���̶���ͬ���� ��
��2�������ڡ��ۻ�Ϻ�ǡ����ȫ��Ӧ����������Һ��������� �ۣ��������������=������
��3����������Һͬ��ϡ��lO������Һ��pH��
�� �ڣ��� �ܣ��� �ޣ��������������=������
��0.01molgL-1CH3COOH��Һ����0.01mo1-1HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01mol-1CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01mol-1HCl��Һ��pH=12��NaOH��Һ��������������Һ��
��1������ˮ�ĵ���̶�������
��2�������ڡ��ۻ�Ϻ�ǡ����ȫ��Ӧ����������Һ���������
��3����������Һͬ��ϡ��lO������Һ��pH��
��
���㣺���������ˮ��Һ�еĵ���ƽ��,pH�ļ���
ר�⣺����ƽ������Һ��pHר��
��������1����������ˮ���룬���������ӵ��δٽ�ˮ���룬���������ӻ��������������Ũ�����ʱ��������ˮ����̶���ȣ�
��2��һˮ�ϰ���������ʣ�pH=12�İ�ˮŨ�ȴ���0.01mol/L�����Ԣڵ�Ũ��С�ڢۣ���Ϻ�ǡ����ȫ��Ӧ�������������ڰ�ˮ��
��3�����������Һ�д��ڵ���ƽ�⣬��ˮϡ�����дٽ�������ʵ��룬��ͬpH��������Һ�У�pH�仯�����ǿ����ʣ��仯С����������ʣ�
��2��һˮ�ϰ���������ʣ�pH=12�İ�ˮŨ�ȴ���0.01mol/L�����Ԣڵ�Ũ��С�ڢۣ���Ϻ�ǡ����ȫ��Ӧ�������������ڰ�ˮ��
��3�����������Һ�д��ڵ���ƽ�⣬��ˮϡ�����дٽ�������ʵ��룬��ͬpH��������Һ�У�pH�仯�����ǿ����ʣ��仯С����������ʣ�
���
�⣺��1����������ˮ���룬�����������ӵ��δٽ�ˮ���룬�٢ڢۢܢ�����ˮ���룬�Ȳ��ٽ�ˮ����Ҳ������ˮ���룬����ˮ�ĵ���̶������Ǣޣ�
����������Ũ�Ⱥͼ�������������Ũ�����ʱ��ˮ�ĵ���̶���ͬ������������Ũ�Ⱥۢ͢�������������Ũ����ȣ�����ˮ�ĵ���̶���ͬ���Ǣڢۢܣ�
�ʴ�Ϊ���ޣ��ڢۢܣ�
��2��һˮ�ϰ���������ʣ�pH=12�İ�ˮŨ�ȴ���0.01mol/L�����Ԣڵ�Ũ��С�ڢۣ���Ϻ�ǡ����ȫ��Ӧ����������ʵ������ڰ�ˮ�����ʵ�������Ϊ���Ũ��С�ڰ�ˮ���������������ڰ�ˮ���ʴ�Ϊ������
��3�����⼸����Һϡ����ͬ�ı���ʱ����ˮϡ�ʹٽ�������ʵĵ��룬���д������̶�С�ڢڣ����Ԣ���������Ũ�ȴ��ڢ٣����Ԣٵ�pH���ڣ�
�ۢ�������������Ũ����ȣ���ˮϡ�ʹٽ�һˮ�ϰ����룬���¢�������������Ũ�ȴ��ڢܣ����Ԣ۵�pH���ܣ�
���м�ˮϡ�ͺٽ�һˮ�ϰ����룬��Һ������������Ũ�ȼ�С������Һ��Ȼ�ʼ��ԣ���
�Ļ����Һ�����ԣ�����pH�ݣ��ޣ�
�ʴ�Ϊ��������������
����������Ũ�Ⱥͼ�������������Ũ�����ʱ��ˮ�ĵ���̶���ͬ������������Ũ�Ⱥۢ͢�������������Ũ����ȣ�����ˮ�ĵ���̶���ͬ���Ǣڢۢܣ�
�ʴ�Ϊ���ޣ��ڢۢܣ�
��2��һˮ�ϰ���������ʣ�pH=12�İ�ˮŨ�ȴ���0.01mol/L�����Ԣڵ�Ũ��С�ڢۣ���Ϻ�ǡ����ȫ��Ӧ����������ʵ������ڰ�ˮ�����ʵ�������Ϊ���Ũ��С�ڰ�ˮ���������������ڰ�ˮ���ʴ�Ϊ������
��3�����⼸����Һϡ����ͬ�ı���ʱ����ˮϡ�ʹٽ�������ʵĵ��룬���д������̶�С�ڢڣ����Ԣ���������Ũ�ȴ��ڢ٣����Ԣٵ�pH���ڣ�
�ۢ�������������Ũ����ȣ���ˮϡ�ʹٽ�һˮ�ϰ����룬���¢�������������Ũ�ȴ��ڢܣ����Ԣ۵�pH���ܣ�
���м�ˮϡ�ͺٽ�һˮ�ϰ����룬��Һ������������Ũ�ȼ�С������Һ��Ȼ�ʼ��ԣ���
�Ļ����Һ�����ԣ�����pH�ݣ��ޣ�
�ʴ�Ϊ��������������
���������⿼����������ʵĵ��뼰pH���㣬����������ʵ����ص��ж���ͬpH����Һ�����ʵ���Ũ�ȹ�ϵ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����ͼ��ʾʵ��װ�õ�K�պϣ������ж���ȷ���ǣ�������


| A��������Zn��a��b��Cu·������ |
| B��Ƭ�̺�׳������������Ũ������ |
| C��Cu�缫�Ϸ�����ԭ��Ӧ |
| D��Ƭ�̺�ɹ۲쵽��ֽb����ɫ |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A����NaAlO2��Һ��ͨ�����CO2��AlO2-+CO2+2H2O�TAl��OH��3��+HCO3- |
| B��Cl2��ˮ�ķ�Ӧ��Cl2+H2O�T2H++Cl-+ClO- |
| C��������Һ��ˮ���е�CaCO3��Ӧ��CaCO3+2H+�TCa2++H2O+CO2�� |
| D��FeCl3��Һ��Cu�ķ�Ӧ��Cu+Fe3+�TCu2++Fe2+ |
 A��B��C��DΪԭ���������������ǰ������Ԫ�أ�Ԫ��Aԭ���������������ڲ��3����Ԫ��B��̬ԭ�Ӻ�����2��δ�ɶԵ��ӣ�Ԫ��C����ۺ���ͼ۴����͵���0��Ԫ��Dλ�����ڱ���B�壮
A��B��C��DΪԭ���������������ǰ������Ԫ�أ�Ԫ��Aԭ���������������ڲ��3����Ԫ��B��̬ԭ�Ӻ�����2��δ�ɶԵ��ӣ�Ԫ��C����ۺ���ͼ۴����͵���0��Ԫ��Dλ�����ڱ���B�壮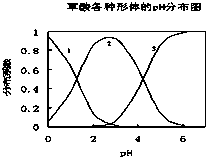 ���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��